- The applicant should be responsible for the product and all information supplied in support of his application for registration of the product
- The applicant should be responsible for updating any information relevant to the product/ application. The NMRA should be informed in a timely manner any change in product information during the course of evaluation, and after product registration, especially if the information pertaining to rejection/ withdrawal, additional data on product quality, effectiveness and safety or current Good Manufacturing Practice (cGMP) compliance of the manufacturers
- The applicant should provide additional documents for renewal of registration, six months before the expiry of certificate of registration
- The marketing authorization holder must assume responsibility for the quality, safety and effectiveness of his products
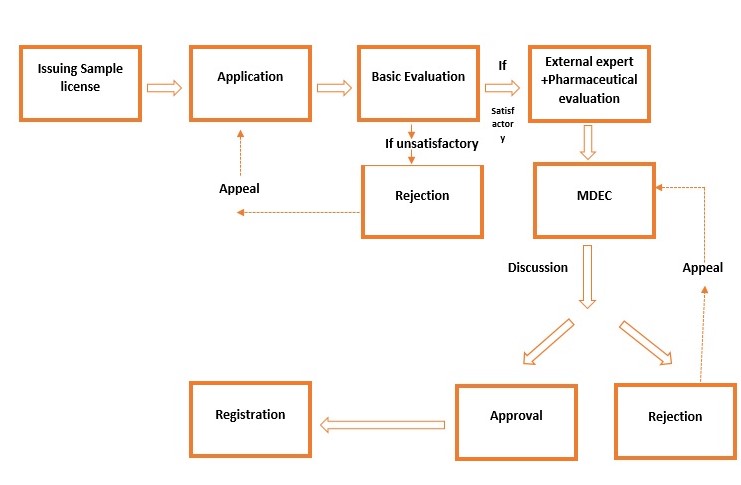
Steps involved for registration of medical devices
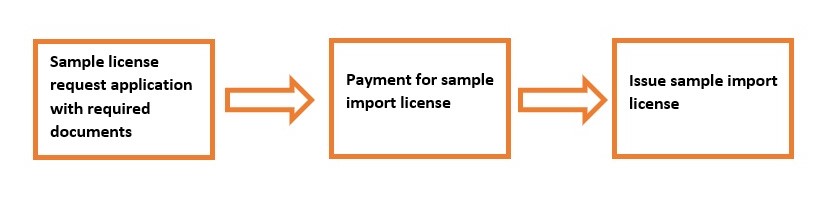
Submission of registration application
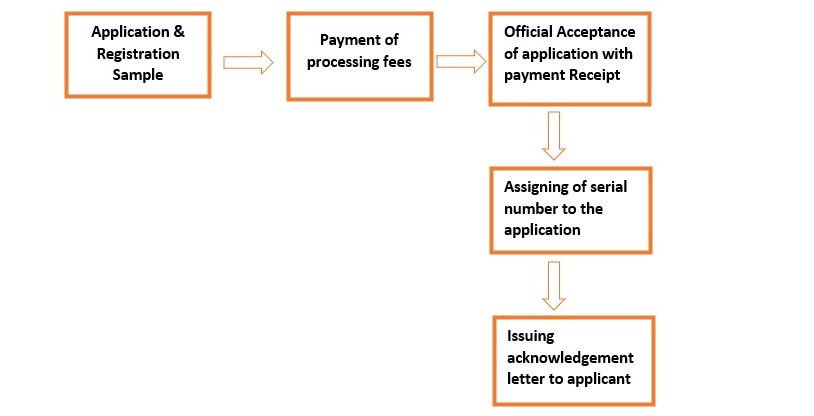
Documents for obtaining sample import license
- Fulfilled application Form C schedule IV of Cosmetics, Devices and Drugs Regulations (The gazette of the Democratic Socialist Republic of Sri Lanka (Extraordinary) No. 378/3 of 1985)
- A copy of business registration certificate of the applicant [should indicate details of the board of directors, secretarial board (Form 48)]
- letter of authorization from the manufacturer appointing the Market Authorization Holder
- Copy Free Sale Certificate of particular product from relevant health authority of country of origin
- Price comparison and CIF price (Separate applications should be submitted for different manufactures)
Application for medical device registration
- The application for registration shall be made along with the required documents in Schedule I, Form A (link to forms) of Cosmetics, Devices and Drugs Regulations (The gazette of the Democratic Socialist Republic of Sri Lanka (Extraordinary) No. 378/3 of 1985)
- Documents should be in English, in a legible font size, printed in one side A4 and submitted in a hard file cover (Box file) and all pages should be numbered from top to bottom and vice versa with an index. Certified English translations should be submitted if the original certificates or licenses issued in any other languages by relevant competent authorities
- The applications for registration are processed only if they are complete and as per specifications
- Separate applications should be made in respect of each device to be registered. [i.e. products containing different specifications, different brands] . Products of foreign manufacturers should be submitted through a Marketing authorization Holder
Submission of applications for Orthopedic Implants and Instruments
This is to inform you that Sri Lanka Orthopedic Association has submit a new guideline for classification of orthopedic implants and instruments for the purpose of submission of applications for sample import license and registration to NMRA (Annex 1). As such you should follow below mention procedure for submission of applications for orthopedic implants and instruments.
Step 1 - Categorized the implants or instruments to main categories
(i) Orthopedic implants or instruments
(ii) Oral Maxillofacial (OMF) implants or instruments
(iii) Neuro implants or instruments
Step 2 - Classification of main group
For orthopedic products separate dossiers to be submitted according to the classification for main six (6) groups.
Step 3 - Classification of sub group
Sub groups intended to register under each main group should be clearly categorized by the manufacturer based on the Free Sale Certificate.
Eg: Main group - Adult plates and screws.
Sub groups - Large fragment locking plates and screws, Mini fragment locking plates and screws, small fragment locking plates and screws.
The NMRA will coordinate with the applicants those who has already submitted registration dossiers for re-arrangement according to the above guideline requirements.
Multiple Applications
- A separate application is required for each product i.e. product containing different specifications or by a different manufacturer shall require a separate application for product registration
- Separate application to be submitted when a medical device consists of different constituents/ components. Each and every component of that system is registered separately.
- Orthopedic system - separate applications should be produced for bone plates, nails, pins, screws
- Dental appliances
- A medical device although the manufacturing process is same and shares a common intended purpose is registered separately
- Condoms with different texture (flavor)
- Syringes with different volumes
- CV catheters, hemodialysis catheters, blood bags (for single, double and triple)
- In vitro diagnostic devices that consist of reagents or article intended to be used in combination to complete a specific intended purpose is registered as a group
- Hematology analyzer with standards, programme and reagents or as separately
- Blood grouping reagent, blood glucose monitoring system with compone
A medical device consisting a collection of devices and has a common intended purpose is registered as a group.
Electro surgical unit with standard accessories (electrodes, electrode holders, leads, Plates, plug adopter)
Anesthesia machine with standard accessories
Nebulizer system
Data requirement for preparation of Medical Device application
General Documents/ Requirements
Fulfilled Schedule I, Form A & Form B
- Copy of sample import license
- Free sale certificate or certificate to foreign government issued from relevant health authority of country of origin and certified by Sri Lankan Embassy of country of origin or foreign affairs
- Fully packed samples (two) of devices in the form that is intended to be marketed (including Lot no., Man. Date, Exp.date, Manufacturer’s & Importers details and when required sufficient quantity for analysis)
- Letter of authorization from the manufacturer appointing the Market Authorization Holder
- List of countries which the device is approved or registered for sale with copies to prove registration status
Technical Documents
Following documents should be submitted in addition to the basic documents where necessary / if available
Final product inspection report (for electro medical equipment and machines) and finished product test report for other products Submit relevant report issued by the manufacturer or third-party laboratory for batch release of the product
Test reports for below mentioned items
- Independent analytical certificates (original report) from Industrial Technology Institute (ITI), Sri Lanka or govt. accreted laboratory in of the country of origin for products which are directly in contact with the blood stream
- Eg: disposable syringes, disposable needles, IV cannulas IV catheters, fistula needles etc
- Test reports are to be submitted according to pharmacopeia standards where the standards are available
- Analytical test reports from Sri Lanka Standard Institute (SLSI) for products feeding bottles, tooth brushes and medical gas cylinders
- Analytical test reports from Industrial Technology Institute (ITI)
- Eg: plasters, gauze, sanitary napkins, bandages, latex condoms, surgical and examination gloves etc.
- In addition, reports from NMQAL may be requested
Material test report for sutures, medical instruments such as forceps, scissors etc.
Certification for quality management system according to ISO 13485 from authorized notified body in order to access the design, development, manufacturing as well as for post marketing monitoring of safety and performance of the manufacturer
CE accreditation from authorized notified body in order to prove the quality assurance system of the product and EC design examination certificate (if applicable)
Stability data for entire shelf life of the finished products should be provided (if applicable)
In addition, following requirements should be fulfilled for Absorbable sutures with the application
- All the samples of absorbable sutures will be kept at the NMRA for six (6) months before sending for evaluation to the relevant consultant
Details of the raw material sources, purchasing details should be provided
Where necessary certificate of approval from relevant authorities should be provided
For radiation emitting devices approval obtained from Atomic energy Authority of Sri Lanka
Certification from the relevant health authority of the country of manufacturer that the product is free from BSE (Bovine Spongiform Encephalopathy) should be obtained for animal derived products
Surgical Catgut
Biological evaluation/biocompatibility test report of medical device as per ISO standards (if applicable)
Risk management analysis as per ISO standard (if applicable)
Recently issued validation report for sterilization process for two commercial batches (if applicable)
Product Label (primary and secondary) Submit original label including following information
Name of product [Approved name and brand name (if any)]
Name and address of the actual manufacturer
Whether the product is sterile and mode of sterilization
Storage conditions specifying the temperature
Manufacturing date, Expiry date and Lot no/ batch no. (if applicable)
Patient information Leaflet
Product information leaflet for the products which are individually handled by the patient in the household should be in both Sinhala and Tamil languages
Eg : Glucometers, Hearing aids, spacer device etc.
Validity Period of registration
The Provisional Registration for a period of one year (or two) will be issued for first time registration and is specified in the certificate
The Full Registration of a product is valid for a period of five years and is specified in the certificate
When additional data are requested, the applicant will have to furnish additional information requested by the authority within 3 months to facilitate further evaluation
If the product is rejected, the market authorization holder will be able to appeal for registration
Renewal of Registration
Application for renewal should be made before six months from the date of expiry of registration
A grace period will extend until the decision is given to the application for renewal
If the requirements for registration are not satisfactory the application will be rejected completely





