I. Preliminary Evaluation (Product Classification) –Valid period (only 1 year)
II. Product Registration- Valid period (1 year, 2 year or 5 years)
Local Agent Profile Creation / Local Manufacturing Profile Creation and obtaining a Preliminary Evaluation Report (Product Classification Report) are mandatory requirements to initiate the product registration process and application submission should be done through eNMRA facility. Before the preliminary evaluation, local agent / local manufacturing profile creation is a main requirement. (via eNMRA platform). Except Preliminary Evaluation (Product Classification) and profile creation all the other applications should be submitted manually.
Currently this process (Local Agent Profile Creation / Local Manufacturing Profile Creation) has been hold temporally due to online system failure and all the submissions should be done manually.
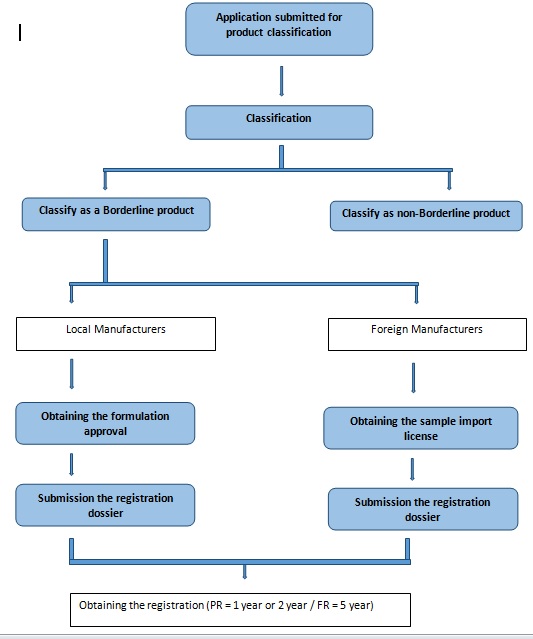
Steps in Borderline product registration:
1. Preliminary evaluation (product classification)
2. Obtaining formulation approval (applicable only for local manufacturers) or sample import license (applicable only for foreign manufacturers)
3. Product registration (Local / Foreign)
Process of registration.
1.0 Required documents for “Preliminary evaluation (Product Classification)” under Borderline Product Category
1. Covering letter including contact details of the local agent (e-mail address, contact numbers, address/s)
2. Copy of Letter of Authorization from the manufacturer which addressed to Director General / CEO , NMRA ((If more than one local agent present for a manufacturer, the list of local agents appointed by the manufacturer and approved product list - for local agents only)
3. Formulation * (Batch manufacturing formula)
4. Valid copy of Free Sale Certificate (FSC) /Certificate of Pharmaceutical product (COPP) / or a certificate to prove the registration status in country of origin (Not applicable for locally manufactured products)
5. Copy of Certificate of Analysis for Finish Product
6. Specimen colored label, Packaging materials (art work acceptable)
7. Legible product information leaflet
8. Patient information leaflet, Promotional materials (If applicable only)
* All applicants who submit applications under Borderline products should submit the composition of unit dose along with the batch manufacturing formula.
Vitamins and minerals should be provided according to the “Guideline on Product categorization Reference details of Vitamins & Elements” publish in the NMRA website under borderline product category. (Units and form of vitamin & minerals)
Examples:
• Elemental –Sodium (Sodium should be given as “Na” but not as “NaCl”)
• Vitamins-Vitamin A (Vitamin A should be given as “ vitamin A” but not as “ retinol’)
• Units- Vitamin D in μg/day, Zn in mg/day)
Refer the Borderline Product Guideline - “Guidance Document on definitions and information for applications submitted under Borderline Product category”
For further Information click here
All noncompliant applications will be rejected with effect from 13th of July 2020.
After the classification process, Classification Report will be issued. Validity period of Preliminary Evaluation Report (Classification Report) is one year and product registration process should be initiated within the validity period of the Classification Report.
2.1 Required documents for obtaining the formulation approval (Only for Local Manufacturers)
1. Preliminary evaluation report (Classification Report)
2. Covering letter
3. Formulation (Master formula & Batch manufacturing formula)
2.2 Required documents for obtaining the Sample Import License (for imported products)
1. Preliminary Evaluation Report (Classification Report) issued by NMRA
2. Application for a License to import a limited quantity of any borderline product(s) for testing, examination, distribution, sample analysis or clinical trial
3. Copy of Business Registration Certificate.
4. Copy of Letter of Authorization from the manufacturer which addressed to Director General / CEO, NMRA (If more than one local agent present for a manufacturer, the list of local agents appointed by the manufacturer and approved product list).
5. Catalogue (If applicable only)
6. Valid Copy of Free Sale Certificate (FSC) /Copy of Certificate of Pharmaceutical Product (COPP)
7. Specimen colored label and packaging materials
3.0 Required documents for product registration
3.1 Local Manufactured products
1. Acknowledgment form – 02 copies
2. Declaration letter
3. Borderline Classification Report issued by NMRA
4. Formulation approval letter – for new registration
5. Previous Registration certificate – for re registration
6. Maximum retail price
7. Business registration certificate
8. Wholesale License (If available).
9. GMP report or GMP certificate for manufacturing site
10. ISO certificate ( If GMP certificate is not available)
11. Manufacturing license
12. Registration certificates of other countries ( If available)
13. Finish product test specifications
14. Certificate of analysis for finished Product ( Original)
15. Certificate of Analysis of Active Pharmaceutical Ingredient (API)
16. API GMP compliance certificates (If applicable)
17. Certificate of Analysis of excipients
18. Manufacturing formula with batch size and master formula with function of each ingredient
19. Manufacturing process with process flow diagram clearly
20. Manufacturing process validation report for three consecutive commercial batches (If applicable only)
21. Analytical validation report (if applicable only)
22. Stability data for 3 commercial batches (Real time and accelerated data) – Original documents ( If applicable only)
23. Specifications & Certificates of Analysis of Packaging materials
24. Product Information Leaflet
25. Patient Information Leaflet (If applicable only)
26. Efficacy data ( If applicable only)
27. Packaging materials- Specimen colored label & six side displayed secondary package (If applicable only)
28. Promotional Material (If applicable only)
29. Two samples
30. Post marketing data (If applicable only)
31. Published Clinical trial data for finish product (If applicable only)
32. Catalogue (If applicable only)
3.2 For Imported Products
1. Acknowledgment form – 02 copies
2. Declaration letter
3. Letter of Authorization from the manufacturer which addressed to Director General / CEO, NMRA (If more than one local agent present for a manufacturer, the list of local agents appointed by the manufacturer and approved product list)
4. Borderline Classification Report issued by NMRA
5. Sample Import License – for new registration
6. Previous Registration certificate – for re registration
7. Maximum retail price
8. Business registration certificate
9. Wholesale License (If available)
10. Valid original of Free Sale Certificate (FSC) /Certificate of Pharmaceutical product (COPP) / or a certificate to prove the registration status in country of origin
11. GMP report or GMP certificate for manufacturing site
12. ISO certificate ( If GMP certificate is not available)
13. Manufacturing license
14. Registration certificates of other countries ( If available only)
15. Finish product test specifications
16. Certificate of analysis for finished Product (Original)
17. Finish product test specifications (If applicable only)
18. Certificate of Analysis of API
19. API GMP compliance certificates (If applicable only)
20. Certificate of Analysis of excipients
21. Manufacturing formula with batch size and master formula with function of each ingredient
22. Manufacturing process with process flow diagram clearly
23. Manufacturing process validation report for three consecutive commercial batches (If applicable only)
24. Analytical validation report (if applicable only)
25. Stability data for 3 commercial batches (Real time and accelerated data) – Original documents – (If applicable only)
26. Specifications & Certificates of Analysis of Packaging materials
27. Product Information Leaflet
28. Patient Information Leaflet (If applicable only)
29. Efficacy data ( If applicable only)
30. Packaging materials- Specimen colored label & six side displayed secondary package (If applicable only)
31. Promotional Material (If applicable only)
32. Two samples
33. Post marketing data (If applicable only)
34. Published Clinical trial data for finish product (If applicable only)
35. Catalogue (If applicable only)
Refer –
• Borderline Product Guideline – “Guidance Document on definitions and information for applications submitted under Borderline Product category”
• F-BPR-01 Assessment form Registration Application
For further Information click here





