Guidance document for local manufacturers producing non- medical face masks
Introduction
A face mask is a device worn over the face to reduce the risk of transmission of pathogens between people, especially those working in healthcare settings. Wearing a homemade cloth mask in the community has not been proven to protect the person who is wearing it. However, the use of a face mask or at least a facial covering can be an additional measure to protect the public from harmful pathogens.
Wearing a mask is another way of covering the mouth and nose to prevent contamination of respiratory droplets from a person to others or landing on surfaces especially when physical distancing is not possible in certain public settings (e.g., grocery shopping, public transport). With the current epidemic situation demand for face masks increased dramatically, and could not be met by the available local production capacity. As a result many others have initiated local manufacturing ventures. However, the local industry has had to face many challenges including lack of specified standards and product specifications as well as testing facilities in Sri Lanka.
Therefore as the national regulatory body, the NMRA identified the need for establishment of standards and specifications for different types of face masks.
This guidance document describes the requirements for different types of face mask and the regulatory procedure that every local manufacture of non- medical face mask should follow in order to ensure a product of good quality and safety are made available to the public.
Consideration for using a face mask
1.Purpose of mask use: the rationale and reason for mask use should be clear - whether it is to be used for source control (used by infected persons) or prevention of COVID-19 (used by healthy persons)
2.Risk of exposure to the COVID-19 virus in the local context:
- The population: current epidemiology about how widely the virus is circulating (e.g., clusters of cases versus community transmission), as well as local surveillance and testing capacity (e.g., contact tracing and follow up, ability to carry out testing).
- The individual: working in close contact with public (e.g., community health worker, cashier).
3.Vulnerability of the person/population to develop severe disease or be at higher risk of death, e.g. people with comorbidities, such as cardiovascular disease or diabetes mellitus, and older people
4.Setting in which the population lives in terms of population density, the ability to carry out physical distancing (e.g. on a crowded bus), and risk of rapid spread (e.g. closed settings, slums, camps/camp-like settings).
5.Feasibility: availability and costs of the mask, and tolerability by individuals.
6.Type of mask: medical mask versus nonmedical mask
Types of Face masks.
There are various types of masks available in all over the world. These may be categorized in to four main types.
1. Non - medical face mask
2. Surgical Face masks
3. Non- medical N95 respirators
4. Surgical N95 Respirators
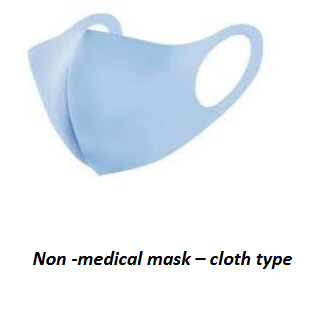
Non - medicalface masks/ Face coverings (Cloth & paper type)
These may be made of cloth or paper and are use as a facial covering for mouth and nose to prevent respiratory droplets of the wearer contaminating the others or landing on surfaces. These types of masks may not be effective in blocking virus particles that may be transmitted by coughing, sneezing or certain medical procedures. They do not provide complete protection from virus particles because of a potential loose fit and the materials used. Certain types of non- medical masks may be reusable depending on the material used.
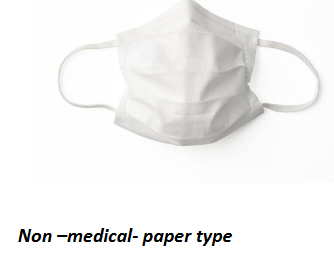
Surgical Face masks/Medical Face Masks/Non PPE Type Face Mask
Surgical masks are single use, fluid-resistant, disposable, and loose-fitting protection devices that create a physical barrier between the mouth and nose of the wearer and the immediate environment but do not achieve a close seal to the wearer's face. Surgical masks should cover both the mouth and the nose, and be secured using the ear loops or ties at the back of the head.

Surgical masks have different grades of filtration and are useful for blocking splashes and large particle droplets or sprays which may occur. They do not provide complete protection from germs and other small particle contaminants. These masks are intended to be worn by health professionals during healthcare procedures.
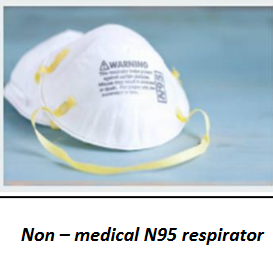
Non- medical respirators / Non- medical N95 respirators/FFR
A filtering face piece respirator (FFR) are designed to form a very close seal around the nose and mouth, protecting the wearer from exposure to airborne particles including pathogenic biological airborne particulates such as viruses and bacteria. N95 respirators have been tested for particulate filtration to ensure they remove a minimum of 95% solid and liquid aerosols that do not contain oil. N95 respirators are a single use items and for industrial use.
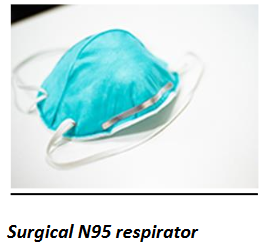
Surgical Respirators/Surgical N95 Respirators/FFR
A filtering face piece respirator (FFR) are with a similar structure and design to standard N95 respirators to protect the wearer from exposure to pathogenic biological airborne particulates, therefore meeting the same testing requirements for a minimum 95% filtration against airborne particulates. Surgical respirators have also been tested for fluid resistance against penetration by synthetic blood under different pressures, such as may occur during certain high risk medical procedures.
Types of masks and respirators — comparison
| Face mask(clothorpapermasks) | Surgical mask | N95 respirator | Surgical N95 respirator | |
| Is it a medical device | No | Yes | No | Yes |
| Medical device class | N/A | Class 1 | N/A | Class 11a |
| Single use | Yes | Yes | Yes | Yes |
| Material | Cloth or Paper | non-woven fiber | electrostatic non-woven polypropylene fiber | electrostatic non-woven polypropylene fiber |
| Purpose | Prevents large particles expelled by the wearer, from reaching the environment. |
Prevents large particles expelled by the wearer when you are ill, from reaching the environment.
To be used as a physical barrier to protect you from large droplets of blood or body fluids. |
Reduces the wearer exposure to very small airborne particles or contaminants.
May not protect against sprays and direct liquid splashes. |
Provides the protection of both a surgical mask and N95 respirator.
To be used as a physical barrier from large droplets of blood or body fluids as well as very small particles (e.g. fine aerosolized droplets), such as those produced by coughing. |
| Fit | Does not fit tightly | Does not fit tightly | Tight fit# | Tight fit# |
| Microbial cleanliness (ISO 11737-1:2018) |
Yes ≤ 30 |
Yes ≤ 30 |
Yes ≤ 30 |
Yes ≤ 30 |
| Bacteria Filtration efficiency | To be tested | Bacterial filtration efficiency above 95%* | Minimum 95%** against particulate aerosols (of 0.3 micron in size) free of oil | Minimum 95%** against particulate aerosols (of 0.3 micron in size) free of oil. |
| Differential Pressure (Delta P) mm H2O/cm2 |
< 4.0 | < 5.0 | < 5.0 | < 5.0 |
Fluid resistance |
Not fluid resistant |
Yes |
Not tested for fluid resistance |
Tested to be fluid-resistant |
| Particulate filtration efficiency | No | No | Particulate filtration efficiency Minimum95%** against particulate aerosols (of 0.3 micron in size) free of oil. | Particulate filtration efficiency Minimum 95%** against particulate aerosols (of 0.3 micron in size) free of oil |
NMRA Requirements for Non -medical masks
Non-medical face masks or face coverings should:
• allow for easy breathing
• fit securely to the head with ties or ear loops
• maintain their shape after washing and drying
• be changed as soon as possible if damp or dirty
• be comfortable and not require frequent adjustment
• be made of at least 2 layers of tightly woven material fabric (such as cotton or linen)
• be large enough to completely and comfortably cover the nose and mouth without gaping
Non-medical masks or face coverings should not:
• be shared with others
• impair vision or interfere with tasks
• be placed on children under the age of 2 years
• be made of plastic or other non-breathable materials
• be secured with tape or other inappropriate materials
• be made exclusively of materials that easily fall apart, such as tissues
• be placed on anyone unable to remove them without assistance or anyone who has trouble breathing
Limitations/or non- medical mask
Homemade or non -medical masks are not medical devices and are not regulated as medical masks and respirators. Their use poses a number of limitations:
• they have not been tested to recognized standards
• the fabrics are not the same as used in surgical masks or respirators
• the edges are not designed to form a seal around the nose and mouth
• they may not provide complete protection against virus-sized particles
• they can be difficult to breathe through and can prevent wearer from getting the required amount of oxygen needed
Procedure for obtaining an approval for Non — medical mask manufactured in
Sri Lanka
Non -medical masks are not medical devices and are not regulated as medical face masks according to the available provisions in the National Medicines Regulatory Authority Act No.05 of 2015.
However according to the powers vested with the authority to ensure the public health, the NMRA will issues a “Letter of Notification” for non- medical type of mask enabling the public to accessible for a product with desired quality and safety.
Required documents for apply for “Letter of Notification”
1. Application form
A person who is intended to obtain the approval from NMRA for Non — medical face mask should submit an application to the authority as per the format in attached Annex I with following documents.
2.Request letter
A request letter should be dully signed by the applicant/manufacturer addressed to Chief, Executive Officer (CEO) of NMRA
3.Finished product test report
The manufacturer should tested the product in an NMRA authorized accredited laboratory for following specifications.
| Test name | Test method/ specification | Sample size | Remark |
|
Physical parameters
|
ASTM D1683-17(R2018) Modified |
To be inquired | Mandatory |
| Bacterial Filtration Efficiency (BFE) % | ASTM F2101 / EN 14683 | 6 Uncontaminated Clean Sealed Samples | Mandatory |
| Microbial cleanliness | ISO 11737 -1 : 2018 | 5 Uncontaminated Clean Sealed Samples | Mandatory |
| Differential Pressure (Delta P) mm H2O/cm2 | EN 14683 : 2019 | 6 Uncontaminated Clean Sealed Samples | Mandatory |
| Formaldehyde Content | ISO 14184 - 1 / AATCC 112 | 2 samples | Mandatory |
| PH Value | ISO 3071 /AATCC 81 | 3 samples | Optional |
| Flame Spread | 16 CFR 1610 | 10 products / 0.25 meter bonded material | Optional |
4.Material confirmation
The manufacturer should confirm the type of material used for the production of mask. A pure Cotton or Linen materials are preferable other than non -woven materials.
5.Product label
The manufacturer should submit a label (Draft is acceptable) of the commercial pack.
|
Following are the labeling requirement should be available in the label for non- medical mask.
|
6.Coloured photograph of the face mask
The coloured photographs of the mask should be available from the front view and from rear sides in one page. (2 copies should be submitted.)


7. Coloured photograph a commercial pack
A coloured photograph of a commercial pack should be attached to the application (2 copies should be submitted.)
8.Business Registration (BR)
Registration of the business with divisional secretarial (reservation document will also be accepted)
Submission of applications
The dully signed application with required documents should be filed in a cardboard file clearly stating the name of the applicant with full address of the manufacturing site and contact numbers on the cover. Documents should be submitted in A4 papers. Applications will be accepted on every Tuesdays and Fridays.





