Procedure of Applying for Waiver of Registration (WOR) for Medicines.
Those who are willing to apply for Waiver of registration for Medicines please be kind enough to submit the properly filled application form and relevant documents to office of Sate Ministry of Production, Supply and Regulations & Pharmaceuticals with effect from 01/05/2022.
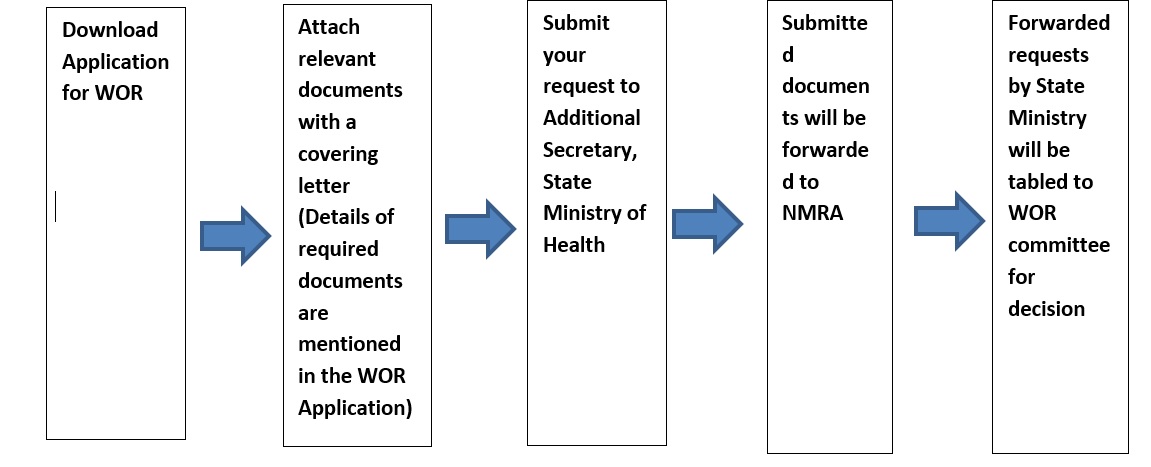
Download WOR Application from here: (https://www.nmra.gov.lk/images/2020/Medicine_Application/Application-for-WOR-medicine.pdf)
Mandatory Documents Required for Waiver of Registration
• Request Letter from the Applicant
• Completed Waiver of Registration Application
• Certificate of Analysis (COA) of the relevant product
• Certificate of Pharmaceutical Product (COPP)
• Commercial invoice / Proforma Invoice
• Product Information Leaflet
• Labels of the product
• Quotation Document
• Purchase order / Award intimation
Note: Incomplete application will not be tabled to WOR Committee.
Decisions of WOR Committee (Medicines) held on 23rd February-2023
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 1/2/2023 | SPC Sri Lanka | PO No: 137069 Indent No: LP/DHS/EP/SA/3645/22 Tender No: DHS/RP/EP/196/2022 | Capecitabine Tablet 500mg USP | Supplier: Pharma Associates Manufacturer : SP Accure Labs Pvt Ltd, India | 250,000 tablets (30's) | LKR 102.2726 per tablet | MSD has indicated that there is a 6 month stock. Hence not permitted. Not permitted. |
| 2 | 14/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/267/2022 Proforma Invoice No: SRL 14 | Etoposide Injection USP 100 mg/5 mL (Etovel 100) | Manufacturer: GLS Pharma Limited, India Local Agent: S S K Pharma Pvt Ltd | 3 000 Vials (1's) | USD 2.40 Per Vial (LKR 876.59) | Five months stock available at MSD. Purchase from registered supplier if more is needed. Not permitted |
| 3 | 14/02/2023 | SPC/Sri Lanka | PO No: 136091 Tender No: DHS/RP/276/22 Indent No: LP/DHS/PH/3637/22 Proforma Invoice No: PFI/JEPL003/22-23 | Cisplatin Injection BP 10 mg/10 mL (JOCISTIN 10) | Supplier: Manufacturer: Jodas Expoim (Pvt) Ltd, India | 225 Vials (1's) | LKR 970.0000 Per Vial | Expeidte Registraton. |
| 4 | 15/02/2023 | SPC/Sri Lanka | PO No: 137296 Indent No: DHS/AMS/503/2022 Tender No: DHS/P/WW/361/2022 Invoice No: SPAL/22-23/EPI/049 | Irinotecan Injection 100 mg/ 5 mL | Supplier/Manufacturer: SP Accure Labs Private Ltd, India Local Agent: Pharma Associates | 330 000 Packs (10x10's) | USD 0.8200 Per CAP of 100 (LKR 2.99/cap) | Purchase from registered supplier. Not permitted. |
| 5 | 14/02/2023 | SPC/Sri Lanka | PO No: 134630 Tender No: DHS/RP/262/2022 Indent No: DHS/PM/478/2022 Commercial Invoice No: UBPL/GEX/2223/0221 | Ifosfamide for Injection USP with Mesna Injection (IFOMID-M-1 g) | Supplier/Manufacturer: United Biotech Pvt Ltd, India Local Agent: George Steuart Health Pvt Ltd | 2 600 Sets (1's) | USD 7.0000 Per Set (LKR 2556.72) | Awaiting MRP decision. Decision Pending |
| 6 | 14/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/21/2022 Export Invoice No: 256 | Melphalan Injection IP 50 mg with solvent (MELFALAX 50) | Supplier: Orexo Bio Pharma (Pvt) Ltd, Sri Lanka Manufacturer: GLS Pharma Ltd, India | 200 Vials (1's) | LKR 16 458.00 Per Vial | Stock available at MSD. Not Permitted. |
| 7 | 14/02/2023 | SPC/Sri Lanka | PO No: 134478 Tender No: DHS/RP/EP/178/2022 Indent No: LP/DHS/EP/PH/3605/2022 Proforma Invoice No: Shipa-47(22-23 | Melphalan Injection IP 50 mg with solvent | Supplier: Slim Pharmaceuticals (Pvt) Ltd, Sri Lanka Manufacturer: Shilpa Medicare Limited, India | 60 Vials (1's) | LKR 39 195.0000 Per Vial | Stock available at MSD. Not Permitted. |
Appeals for the Already Issued WORs
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Previous Decision of WOR Committee | Decision of WOR Committee |
| 8 | 29/12/2022 | SPC/Sri Lanka | Proforma Invoice No: PFI195 | Anastrozole Tablets 1mg | Supplier: Imperial Life Sciences (Pvt) Ltd Manufacturer: Globela Pharma (Pvt) Ltd | 167 000 Tablets (100's) | LKR 9.8345 Per Tablet | Registered products have been over looked for unregistered product. MRP of registered product is below NMRA determined MRP. Purchase from registered product. Not permitted. | No change in decison. Not permitted. |
| 9 | 20/01/2023 | SPC Sri Lanka | PO No: 134427 Indent No: LP/DHS/EP/SA/3599/22 Tender No: DHS/RP/EP/187/2022 | Trastuzumab Powder for Solution for Infusion 440 mg (HERTICAD) | Supplier: Advitec International (Pvt) Ltd Manufacturer: Biocad Closed Joint Stock Company, Russia | 3 000 Vials | LKR 63450.0000 Per Vial | Purchase from registered products. Not permitted. | Not recommended by Oncologists till the product is registered. Not permitted. |
| 10 | 1/2/2023 | SPC Sri Lanka | Tender No: DHS/RP/151/2022 | Ifosamide for Injection USP 1gm with Mesna Injection 200mg/2ml | Local Agent: Imperial Life Sciences (Pvt) Ltd Manufacturer: Health Biotech Ltd, India | 800 Sets (03 amps per set) | USD 8.85 Per Set (LKR 3232.42) | Both manufacturer & local agent not registered with NMRA. MSD has 5 months stock. Not permitted. | Stand with previous decision. Not permitted. |
| 11 | 6/2/2023 | SPC/Sri Lanka | PO No: 137246 Indent No: LP/DHS/EP/MM/3649/2022 Tender No: DHS/RP/EP/304/2022 Proforma Invoice No: PA/PI/SPC/016/22-23 | Propofol Injection Emulsion USP 500 mg/50 mL (PROPO SPAL 500) | Supplier/Local Agent: Pharma Associates Manufacturer: SP Accure Labs Private Limited, India | 25 000 Ampoules (1's) | LKR 1290.0000 Per Ampoule | Manufacturer not registered. Product has a narrow therapeutic index. Not permitted. | After checking the manufacturing plant approval by NMRA of EU GMP which they have till end of 2023 due to Covid the WOR committee is approving the WOR for Propofol Manafactured by SP Accure Labs, India. Permitted. |
| 12 | 6/2/2023 | SPC/Sri Lanka | PO No: 134506 Indent No: LP/DHS/EP/MM/3616/2022 Tender No: DHS/RP/EP/197/2022 Proforma Invoice No: 157 | Gemcitabine Injection USP 200 mg/Vial (As lyophilized) (GEMDN 200) | Supplier/Local Agent: Yaden International (Pvt) Ltd Manufacturer: Biozenta (Pvt) Ltd, India | 750 Vials (1's) | 4,900.00 LKR/Vial | Manufacturer not registered. A registered product with lapsed registration was permitted today. Not permitted. | No change in decision. Not permitted. |
| 13 | 1/2/2023 | SPC Sri Lanka | PO No: 135223 Indent No: LP/DHS/MM/3634/2022 Tender No: DHS/RP/256/2022 Proforma Invoice No: PI014SS | Propofol 1% Injection for Intravenous infusion | Supplier/Local Agent: Unicura International (Pvt) Ltd Manufacturer: Kwality Pharmaceuticals Ltd, India | 12,500 Ampoules (1's) | LKR 774.20 per ampoule | Kwality has had several quality issues. NMRA has requested GMP inspection. Not Permitted. | Contacted anaesthetics: not to purchase owing to quality issues. Not Permitted. |
Decisions of WOR Committee (Medicines) held on 2nd March 2023
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 21-12-2022 | SPC/AD/292/2022 | Pantoprazole for IV Injection 40mg | Cadila Healthcare Ltd, India | 12000 vials | 0.65 USD/Vial (C&F) (LKR 233.09) | Site change under same legal entity. Permitted. | |
| 2 | 7/2/2023 | Unicura International (Pvt) Ltd | Proforma Invoice No: MM/07/22 | Tobramycin Sulphate USP 0.3% w/v Dexamethasone Sodium Phosphate IP 0.05% w/v Chlorine Solution IP 0.02% v/v Eye/ear drops (Tobra D) | Manufacturer: Laborate Pharmaceuticals India Ltd, India | 2001 Packs (5 mL) | USD 0.82 Per Pack (LKR 299.50) | Not an essential medicine, manufacturer has quality issues. Not permitted. |
| 3 | 7/2/2023 | Unicura International (Pvt) Ltd | Proforma Invoice No: MM/06/22 | Tramadol Hydrochloride Injection 100mg/2ml | Manufacturer: Laborate Pharmaceuticals India Ltd, India | 12500 Ampoules (2ml) | Purchase from registered product. Not permitted. | |
| 4 | 1/2/2023 | Unicura International (Pvt) Ltd | Proforma Invoice No: MM/04/22 | Amphotericin B Injection 50mg | Manufacturer: Affy Parenterals, India | 250 vials | USD 4.65 (LKR 1698.39) | Both manufacturer & MAH not registered. Not permitted. |
| 5 | 1/2/2023 | Unicura International (Pvt) Ltd | Proforma Invoice No: MM/08/22 | Chlorambucil Tablets 2mg | Manufacturer: Celon Laboratories Pvt Ltd, India | 67 packs | USD 36/ Pack (30's) LKR 438.29 per tablet) | As LA is not registered for Celon laboratories. Not permitted |
| 6 | 1/2/2023 | Unicura International (Pvt) Ltd | Proforma Invoice No: MM/05/22 | Natamycin Opthalmic Suspension 5% | Manufacturer: Hanuchem Laboratories, India | 1250 packs | USD 1.25 ( LKR 456.56) | Both manufacturer & MAH not registered. Not permitted. |
| 7 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Betamethasone Valerate Cream BP 0.1% w/w (15g) | Manufacturer: Hovid Berhad, Malaysia | 800 Tubes | USD 0.70 (LKR 255.67) | Permitted as it is a registered product. |
| 8 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Metformin Tablets BP 500mg | Manufacturer: Hovid Berhad, Malaysia | 2000 boxes | USD 1.34/ Pack (10x10's) (LKR 4.89 per tablet) | Permitted as it is a registered product. |
| 9 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Metformin Tsablets BP 850mg | Manufacturer: Hovid Berhad, Malaysia | 150 boxes (10x10's) | USD 2.05/ Pack (10x10's) (LKR 7.48) | Permitted as it is a registered product. |
| 10 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Fluconazole Capsules 150mg | Manufacturer: Hovid Berhad, Malaysia | 2600 boxes (1x4's) | USD 0.80 / Pack (1x4's) (LKR 73.05) | Permitted as it is a registered product. |
| 11 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Fusidic acid cream BP 2% w/w(15g) | Manufacturer: Hovid Berhad, Malaysia | 1800 Tubes | USD 1.03 (LKR 376.20) | Permitted as a registered product. |
| 12 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Fusidic acid 2% w/w and Hydrocortisone acetate 1% w/w cream (15g) | Manufacturer: Hovid Berhad, Malaysia | 1000 Tubes | USD 0.85 (LKR 310.46) | Permitted as it is a registered product. |
| 13 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Amlodipine Besilate Tablets 5 mg (HOVSAC 5) | Hovid Berhad, Malaysia | 300 Packs (10x10's) | USD 0.0184 Per Tablet (LKR 6.72) | Permitted as it is a registered product. |
| 14 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Itraconazole Capsules 100 mg (INOX 100) | Hovid Berhad, Malaysia | 5 000 Packs (7x4's) | USD 0.1729 Per Capsule (LKR 63.15) | Permitted as a registered product. |
| 15 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Montelukast Tablets 10 mg (MONTELAIR 10) | Hovid Berhad, Malaysia | 1 000 Packs (3x10's) | USD 0.08 Per Tablet (LKR 29.22) | Permitted as it is a registered product. |
| 16 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Betamethasone Valerate with Neomycin Sulphate Cream (NEO -BETASONE Cream) | Hovid Berhad, Malaysia | 500 Tubes (15 g) | USD 0.71 Per Tube (LKR 259.32) | Permitted as it is a registered product. |
| 17 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Miconazole Nitrate Cream BP 2% (TINAZOL Cream, 2%) | Hovid Berhad, Malaysia | 1 500 Tubes (15 g) | USD 0.65 Per Tube (LKR 237.41) | Permitted as it is a registered product. |
| 18 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Aciclovir Tablets BP 400 mg (VIREST 400) | Hovid Berhad, Malaysia | 100 Packs (5x5's) | USD 0.0976 Per Tablet (LKR 35.66) | Permitted as it is a registered product. |
| 19 | 8/2/2023 | Mansel (Ceylon) (Pvt) Ltd | Invoice No: PROE 258661 | Aciclovir Cream BP 5% w/w (VIREST CREAM 5%) | Hovid Berhad, Malaysia | 100 Tubes (5 g) | USD 0.66 Per Tube (LKR 241.13) | Registered product. Permitted |
| 20 | 13/02/2023 | Breathfree Lanka (Pvt) Ltd | Proforma Invoice No: BFL/22/285 | Oral Rehydration Salts IP Powder 21 g (Prolyte (Orange Flavour)) | Manufacturer: ACME Diet Care (Pvt) Ltd, India | 128 000 Sachets (40 Sachets (21 g)/ Pack) | USD 0.0026 Per Sachet (LKR 0.95) | Permitted as the LA is registered & no products are available in the market. |
| 21 | 13/02/2023 | A Baur & Co. Pvt Ltd | Proforma Invoice No: 1302 | Glimepiride Tablets IP 1mg | Sanofi India Ltd- Goa site | 6000 packs (pack size-2x30's) | USD 2.03 per pack (LKR 12.35 per tablet) | Register product. Not permitted. |
| 22 | 16/02/2023 | Breathe Free Lanka (Pvt) Ltd | Proforma Invoice No: BFL/23/1 | Warfarin Tablets BP 5 mg (WARF-5) | Manufacturer: Cipla Ltd, Malpur Solan, HP 173205, India Local Agent: Breathe Free Lanka (Pvt) Ltd | 10 642 + 10 754 + 10731 Packs (10x10's) | USD 0.0252 Per Tablet (LKR 9.21) | Permitted as the product is registered |
| 23 | 16/02/2023 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: 44 | Pantoprazole for IV Injection 40 mg (PANTODAC IV) | Manufacturer: Sakar Healthcare Limited, India Importer: SPC/Sri Lanka Local Agent: Sunshine Healthcare Lanka Ltd | 10 000 Vials (1's) | USD 1.22 Per Vial (LKR 445.72) | Site change, but price is higher than the price of the application in the 1st application. Not permitted. |
| 24 | 16/02/2023 | Siba Holdings (Pvt) Ltd | Proforma Invoice No: PI/0022/2022-23 PO No: 111041 Indent No: DHS/IN/716/20 Tender No: DHS/RP/458/2020 | Gabapentin Capsules USP 100 mg (RAVASTAL 100) | Stallion Laboratories (Pvt) Ltd, India | 15 000 Packs (10x10's) | USD 0.0096 Per Capsule (LKR 3.51) | Permitted only for this consignment. Register for future. |
| 25 | 20/02/2023 | SPC/Sri Lanka | Proforma Invoice No: KW-754/22 Tender No: DHS/RP/236/2021 | Lorazepam Injection 4mg/ml | Manufacturer: Kwality Pharmaceuticals Ltd, India Supplier: Orexo Bio Pharma Pvt Ltd, Wattala | 3750 Ampoules | USD 4.124 per ampoule (LKR 1505.39) [USD 41.24 per pack of 10's] | Several quality failures. Not apprvoed. College of psychatrists does not recommend. Not permitted. |
| 26 | 20/02/2023 | SPC/Sri Lanka | Indent No: LP/DHS/EP/DA/3617/2022 Tender No: DHS/RP/EP/186/2022 PO No: 134547 | Sunitinib Malate Capsules 50mg | Manufacturer: Boizenta Lifesciences Pvt Ltd, India Supplier: Yaden International Pvt Ltd | 4500 Capsules | LKR 4900 per capsule | Decision Pending |
| 27 | 20/02/2023 | SPC/Sri Lanka | Indent No: DHS/WD/616/19 Tender No: DHS/P/WW/652/19 PO No: 48504 | Linezolid IV Injection 200mg/ 100ml (Lizolid IV) | Manufacturer/ Supplier: Glenmark Pharmaceuticals Ltd, India Local Agent: Sunshine Healthcare Lanka Ltd | 5500 Packs (1's) | USD 2.48 (LKR 906.05) | Product registered. Label accepted. Permitted. |
| 28 | 21/02/2023 | CIC Holding PLC | Proforma Invoice No: CCL/EPI/22-23/01/118 | Tamsulosin HCL 0.4 mg + Dutasteride 0.5 mg Capsule (Maxflow-D Capsule 0.4/0.5 mg) | CCL Pharmaceuticals Pvt Ltd, Pakistan | 2080 Packs (30's) [2000 commercial packs + 80 Physian samples ] | USD10.760/ pack 30's ( LKR 130.14 per capsule) | Expedite registration. Not permitted. |
| 29 | 22/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/257/2022 Indent No: LP/DHS/MM/3632/2022 Proforma Invoice: P1015SS | Propofol 1% Injection BP/IP for intravenous infusion 20ml | Manufacturer: Kwality Pharmaceuticals Ltd, India Supplier: Unicura International Pvt Ltd, Dehiwala Local Agent: Unicura International Pvt Ltd, Dehiwala | 18,000 Ampoules | USD 0.92 (LKR 333.81) | Not permitted as there are several quality failures. |
| 30 | 22/02/2023 | SPC/Sri Lanka | Tender No: DHS/P/WW/775/21 | Colecalciferol Tablets BP 5000 IU | Manufacturer/ Supplier : MCW Healthcare Pvt Ltd, India Local Agent : Innogen Lanka Pvt Ltd | 250,000 Tablets | USD 0.039 (LKR 14.15) | Purchase from registered supplier. Also, not in essential list. Not permitted. |
| 31 | 22/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/EP/308/2022 Indent No: LP/DHS/EP/MM/3653/2022 Proforma invoice No: MLL/YIPL/PFI-42/2022-23 | Ergometrine Injection 500mcg/ 1ml | Manufacturer: Mercury Laboratories Ltd, India Supplier/ Local Agent: Yaden International Pvt Ltd | 3000 Ampoules | LKR 670.00 per Ampoule | To get opinion from College of Obstetricians. Decision pending. |
| 32 | 28/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/165/2022 Invoice No: PFI 235 | Etoposide Capsule USP 50mg | Manufacturer: Globela Pharma Pvt Ltd, India Supplier/ Local Agent: Imperial Lifescience Pvt Ltd | 1600 Capsules | LKR 489.50 per capsule | Request to register 50 mg of needed. 50 mg has been removed from priority list. Not Permitted. |
| 33 | 28/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/264/20212 Proforma Invoice: PFI/JEPL007/22-23 | Oxaliplatin for Injection USP 100mg 50ml | Manufacturer:Jodas Expoim Pvt Ltd, India Supplier: Softcare International Pvt Ltd | 4000 Vials | USD 10.20 (LKR 3700.93) | Buy from Zydus as it was a registered product. Not permitted. |
| 34 | 28/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/470/2020 Invoice No: PFI21061 | Chlorambucil Tablet BP 2 mg | Manufacturer: Bruck Pharma Pvt Ltd, India Supplier: Eureka Life Sciences Pte Ltd, Singapore Local Agent: Yaden International Pvt Ltd | 32,000 Tablets | USD 1.66 (LKR 602.31) | Private market price is LKR 430.33. The price in this application is LKR 602.31. Recommend to negotiate price. Decision pending. |
| 35 | 28/02/2023 | Breathe Free Lanka (Pvt) Ltd | Proforma Invoice No: BFL/22/245 | Warfarin Sodium Tablets IP 5mg (WARF-5) | Manufacturer: Cipla Ltd, India Local Agent: Breathe Free Lanka Pvt Ltd | 183 Packs (Pack size 20x3x30's) | USD 45 / Pack (LKR 9.07/ Tablet) | Permitted as registration is valid. Package is acceptable. |
| 36 | 28/02/2023 | Breathe Free Lanka (Pvt) Ltd | Proforma Invoice No: BFL/22/264 | Ciprofloxacin Hydrochloride Tablets IP 250mg (Ciplox-250) | Manufacturer: Cipla Ltd, India Local Agent: Breathe Free Lanka Pvt Ltd | 2158 Packs (Pack size 10x7x10's) | USD 14.7 / Pack ( LKR 7.61) | Registered product. Packaging acceptable. Permitted |
| 37 | 2/3/2023 | SPC/Sri Lanka | PO No: 136380 Indent No: DHS/PH/495/2022 Tender No: DHS/RP/275/2022 | Melphalan Tablets USP 2 mg (Melfalax 2) | Manufacturer & Supplier: GLS Pharma (Pvt) Ltd, India Local Agent: SSK Pharma (Pvt) Ltd | 4000 Tablets (Pack size: 25's) | USD 0.76 Per Tablet | No registered product. Approved by oncologist Dr. Damayanthi Peiris. Permitted. |
WOR for Oncology Products (Decision Pending by the WOR committe held on 23rd February 2023
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 14/02/2023 | SPC/Sri Lanka | PO No: 134630 Tender No: DHS/RP/262/2022 Indent No: DHS/PM/478/2022 Commercial Invoice No: UBPL/GEX/2223/0221 | Ifosfamide for Injection USP with Mesna Injection (IFOMID-M-1 g) | Supplier/Manufacturer: United Biotech Pvt Ltd, India Local Agent: George Steuart Health Pvt Ltd | 2 600 Sets (1's) | USD 7.0000 Per Set (LKR 2556.72) | Awaiting MRP decision. Decision pending |
| 2 | 14/02/2023 | SPC/Sri Lanka | PO No: 136091 Tender No: DHS/RP/276/22 Indent No: LP/DHS/PH/3637/22 Proforma Invoice No: PFI/JEPL003/22-23 | Cisplatin Injection BP 10 mg/10 mL (JOCISTIN 10) | Supplier: Softcare International (Pvt) Ltd Manufacturer: Jodas Expoim (Pvt) Ltd, India | 225 Vials (1's) | LKR 970.0000 Per Vial | Expeidte Registraton. MRP by NMRA is LKR 924.29. Purchase at the MRP. Decision Pending. |
| 3 | 14/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/21/2022 Export Invoice No: 256 | Melphalan Injection IP 50 mg with solvent (MELFALAX 50) | Supplier: Orexo Bio Pharma (Pvt) Ltd, Sri Lanka Manufacturer: GLS Pharma Ltd, India | 200 Vials (1's) | LKR 16 458.00 Per Vial | Stock available at MSD. Not permitted. |
| 4 | 14/02/2023 | SPC/Sri Lanka | PO No: 134478 Tender No: DHS/RP/EP/178/2022 Indent No: LP/DHS/EP/PH/3605/2022 Proforma Invoice No: Shipa-47(22-23 | Melphalan Injection IP 50 mg with solvent | Supplier: Slim Pharmaceuticals (Pvt) Ltd, Sri Lanka Manufacturer: Shilpa Medicare Limited, India | 60 Vials (1's) | LKR 39 195.0000 Per Vial | Stock available at MSD. Not permitted. |
Oncology list discussed on 9th March 2023
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Previous Decision of WOR Committee (16.02.2023) | Decision of WOR Committee (23.02.2023) | Decision of WOR Committee (02.03.2023) | Decision of WOR Committee (09.03.2023) |
| 1 | 29/12/2022 | SPC/Sri Lanka | Proforma Invoice No: PFI195 | Anastrozole Tablets 1mg | Supplier: Imperial Life Sciences (Pvt) Ltd Manufacturer: Globela Pharma (Pvt) Ltd | 167 000 Tablets (100's) | LKR 9.8345 Per Tablet | Registered products have been over looked for unregistered product. MRP of registered product is below NMRA determined MRP. Purchase from registered product. Not permitted. | No change in decison. Not permitted. | No change in decison. Not permitted. | |
| 2 | 5/12/2022 | SPC/Sri Lanka | DHs/RP/EP/188/2022 | Interferon Beta 1a Injection 44mcg/0.5ml | Slim Pharmaceuticals (Pvt) Ltd Manufacturer CinnaGen Co-Iran | 3,000 PFSY | 14,300.00 LKR/per PFSY | Approved by the 74 th MEC | |||
| 3 | 20/01/2023 | SPC Sri Lanka | PO No: 134427 Indent No: LP/DHS/EP/SA/3599/22 Tender No: DHS/RP/EP/187/2022 | Trastuzumab Powder for Solution for Infusion 440 mg (HERTICAD) | Supplier: Advitec International (Pvt) Ltd Manufacturer: Biocad Closed Joint Stock Company, Russia | 3 000 Vials | LKR 63450.0000 Per Vial | Purchase from registered products. Not permitted. | No change in decison. Not permitted. | No change in decison. Not permitted. | |
| 4 | 1/2/2023 | SPC Sri Lanka | PO No: 137069 Indent No: LP/DHS/EP/SA/3645/22 Tender No: DHS/RP/EP/196/2022 | Capecitabine Tablet 500mg USP | Supplier: Pharma Associates Manufacturer : SP Accure Labs Pvt Ltd, India | 250,000 tablets (30's) | LKR 102.2726 per tablet | MSD has indicated that there is a 6 month stock. Hence not permitted. Not permitted. | MSD has confirmed that there is 5 months stock available. Permitted with write request of MSD | ||
| 5 | 1/2/2023 | SPC Sri Lanka | PO No: 135223 Indent No: LP/DHS/MM/3634/2022 Tender No: DHS/RP/256/2022 Proforma Invoice No: PI014SS | Propofol 1% Injection for Intravenous infusion | Supplier/Local Agent: Unicura International (Pvt) Ltd Manufacturer: Kwality Pharmaceuticals Ltd, India | 12,500 Ampoules (1's) | LKR 774.20 per ampoule | Kwality has had several quality issues. NMRA has requested GMP inspection. Not Permitted. | Checked anaesthetics. therefore not to purchase owing to quality issue. | Kwality has had several quality issues. NMRA has requested GMP inspection. Not Permitted. | |
| 6 | 1/2/2023 | SPC Sri Lanka | Tender No: DHS/RP/151/2022 | Ifosamide for Injection USP 1gm with Mesna Injection 200mg/2ml | Local Agent: Imperial Life Sciences (Pvt) Ltd Manufacturer: Health Biotech Ltd, India | 800 Sets (03 amps per set) | USD 8.85 Per Set (LKR 3232.42) | Both manufacturer & local agent not registered with NMRA. MSD has 5 months stock. Not permitted. | Stand with previous decision. Not permitted. | Stand with previous decision. Not permitted. NMRA has already issued import license to the Grorge steuart pvt Ltd for the same product | |
| 7 | 1/2/2023 | SPC Sri Lanka | DHS/SD/491/2022 | Decarbazine for Injection USP 200 mg | United Biotec Pvt ltd, Georgr Steuart Pvt Ltd | 1500 vials | Approved () | ||||
| 8 | 6/2/2023 | SPC/Sri Lanka | PO No: 134506 Indent No: LP/DHS/EP/MM/3616/2022 Tender No: DHS/RP/EP/197/2022 Proforma Invoice No: 157 | Gemcitabine Injection USP 200 mg/Vial (As lyophilized) (GEMDN 200) | Supplier/Local Agent: Yaden International (Pvt) Ltd Manufacturer: Biozenta (Pvt) Ltd, India | 750 Vials (1's) | 4,900.00 LKR/Vial | Manufacturer not registered. A registered product with lapsed registration was permitted today. Not permitted. | No change in decision. Not permitted. | No change in decision. Not permitted. | |
| 9 | 6/2/2023 | SPC/Sri Lanka | PO No: 137246 Indent No: LP/DHS/EP/MM/3649/2022 Tender No: DHS/RP/EP/304/2022 Proforma Invoice No: PA/PI/SPC/016/22-23 | Propofol Injection Emulsion USP 500 mg/50 mL (PROPO SPAL 500) | Supplier/Local Agent: Pharma Associates Manufacturer: SP Accure Labs Private Limited, India | 25 000 Ampoules (1's) | LKR 1290.0000 Per Ampoule | Manufacturer not registered. Product has a narrow therapeutic index. Not permitted. | After checking the manufacturing plant approval by NMRA of EU GMP which they have till end of 2023 due to Covid the WOR committee is approving the WOR for Propofol Manafactured by SP Accure Labs, India. Permitted. | - | - |
| 10 | 21210 | DHS | PO No: 134630 Tender No: DHS/RP/262/2022 Indent No: DHS/PM/478/2022 Commercial Invoice No: UBPL/GEX/2223/0221 | Ifosfamide for Injection USP with Mesna Injection (IFOMID-M-1 g) | Supplier/Manufacturer: United Biotech Pvt Ltd, India Local Agent: George Steuart Health Pvt Ltd | 2 600 Sets (1's) | USD 7.0000 Per Set (LKR 2556.72) | Awaiting MRP decision. Decision Pending | Awaiting MRP decision. Decision pending | Awaiting MRP decision. ( Please note that shipment clearance has been granted for the previous certificate) | |
| 11 | 15/02/2023 | SPC/Sri Lanka | PO No: 137296 Indent No: DHS/AMS/503/2022 Tender No: DHS/P/WW/361/2022 Invoice No: SPAL/22-23/EPI/049 | Irinotecan Injection 100 mg/ 5 mL | Supplier/Manufacturer: SP Accure Labs Private Ltd, India Local Agent: Pharma Associates | 330 000 Packs (10x10's) | USD 0.8200 Per vial | - | Purchase from registered supplier. Not permitted. | Purchase from registered supplier. Not permitted. | |
| 12 | 8/2/2023 | Irinotecan Injection 40 mg/ 2 mL | Softcare Internationl pvt Ltd | 750 | Shipment clearance has been granted. | ||||||
| 13 | 14/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/21/2022 Export Invoice No: 256 | Melphalan Injection IP 50 mg with solvent (MELFALAX 50) | Supplier: Orexo Bio Pharma (Pvt) Ltd, Sri Lanka Manufacturer: GLS Pharma Ltd, India | 200 Vials (1's) | LKR 16 458.00 Per Vial | Stock available at MSD. Not Permitted. | Stock available at MSD. Not Permitted. This used for the BMT patients and we need quality products with past experience. | ||
| 14 | 14/02/2023 | SPC/Sri Lanka | PO No: 134478 Tender No: DHS/RP/EP/178/2022 Indent No: LP/DHS/EP/PH/3605/2022 Proforma Invoice No: Shipa-47(22-23 | Melphalan Injection IP 50 mg with solvent | Supplier: Slim Pharmaceuticals (Pvt) Ltd, Sri Lanka Manufacturer: Shilpa Medicare Limited, India | 60 Vials (1's) | LKR 39 195.0000 Per Vial | Stock available at MSD. Not permitted. | Stock available at MSD. Not permitted. | Stock available at MSD. Not Permitted. This used for the BMT patients and we need quality products with past experience. | |
| 15 | 14/02/2023 | SPC/Sri Lanka | PO No: 136091 Tender No: DHS/RP/276/22 Indent No: LP/DHS/PH/3637/22 Proforma Invoice No: PFI/JEPL003/22-23 | Cisplatin Injection BP 10 mg/10 mL (JOCISTIN 10) | Supplier: Softcare International (Pvt) Ltd Manufacturer: Jodas Expoim (Pvt) Ltd, India | 225 Vials (1's) | LKR 970.0000 Per Vial | - | Expedite Registration | Expeidte Registraton. MRP by NMRA is LKR 924.29. Purchase at the MRP. Decision Pending. | Permitted for this procurment onlyas a product has been evaluated by the NMRA.Purchase at the MRP |
| 16 | 28/02/2023 | SPC/Sri Lanka | Tender No: DHS/RP/165/2022 Invoice No: PFI 235 | Etoposide Capsule USP 50mg | Manufacturer: Globela Pharma Pvt Ltd, India Supplier/ Local Agent: Imperial Lifescience Pvt Ltd | 1600 Capsules | LKR 489.50 per capsule | Strength is not registerd | Request to register 50 mg of needed. 50 mg has been removed from priority list. | Request to register 50 mg of needed. 50 mg has been removed from priority list of MSD as suggested by the college of oncologist. | |
| 17 | 14/02/2023 | SPC/Sri Lanka | Dasatinib Tablets | Shipment clearance has been granted. | |||||||
| 18 | 20/02/2023 | SPC/Sri Lanka | Indent No: LP/DHS/EP/DA/3617/2022 Tender No: DHS/RP/EP/186/2022 PO No: 134547 | Sunitinib Malate Capsules 50mg | Manufacturer: Boizenta Lifesciences Pvt Ltd, India Supplier: Yaden International Pvt Ltd | 4500 Capsules | LKR 4900 per capsule | - | - | Decision pending | Permitted as there is no registered product and MSD stocl level is low |
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 20/12/2022 | SPC Sri Lanka | Tender No: DHS/RP/54/2022 | Ethambutol Tablets 400 mg | Supplier: Softcare International (Pvt) Ltd Manufacturer: Concept Pharmaceuticals, India | 100 000 Tablets (10x10's) | LKR 57.00 Per Tablet | Permitted as no registered product & manufacturer & local agent are both registered with NMRA. Permitted |
| 2 | 22/12/2022 | SPC/Sri Lanka | Proforma invoice No: 90006805 Tender No: RES/08/04/C/2020 Indent No- SPC/AD/257/2020 | Cefuroxime Axetil Tablets 250mg | Manufacturer/Supplier: Glenmark Pharmaceuticals Ltd, India Local Agent: Sunshine Healthcare Lanka Ltd | 24,000 packs of 5x10's Alu-Alu strip pack | LKR 36.96 Per Tablet | Given as it is a registered product & needs approval for site change only. Permitted. |
| 3 | 23/12/2022 | SPC/Sri Lanka | Proforma Invoice No: PFI194 | Capecitabine Tablets USP 500 mg (GLOCAPX 500) | Supplier: Imperial Life Sciences (Pvt) Ltd Manufacturer: Globela Pharma (Pvt) Ltd | 83 000 Tablets (10's) | USD 0.299 Per Tablet (LKR 109.29) | Approved as no registered product & price is acceptable. Manufacturer is registered with NMRA. Local agent has also applied for registration. Permitted. |
| 4 | 29/12/2022 | SPC/Sri Lanka | Proforma Invoice No: PFI195 | Anastrozole Tablets 1mg | Supplier: Imperial Life Sciences (Pvt) Ltd Manufacturer: Globela Pharma (Pvt) Ltd | 167 000 Tablets (100's) | LKR 9.8345 Per Tablet | Registered products have been over looked for unregistered product. MRP of registered product is below NMRA determined MRP. Purchase from registered product. Not permitted. |
| 5 | 30/12/2022 | Diabetes Association of Sri Lanka | Invoice No : 80187464 | Glargine Insulin (5 cartridges/pack), Humulin 70/30 Insulin (5 cartridges/pack),Humulin Regular (5 cartridges/pack), Humulin NPH (5 cartridges/pack), Huma pen Ergo II | Glargine Insulin - 234 packs (5 cartridges/pack), Humulin 70/30 Insulin- 1194 packs(5 cartridges/pack),Humulin Regular - 264 packs (5 cartridges/pack), Humulin NPH 60 Packs (5 cartridges/pack), Huma pen Ergo II - 19 | Recombinant Insulin Glargine 100U/ml - $0.18, Insulin Human Isophane- 70/30 - $ 0.27, Insulin Human Regular 100u/ml- $0.12, Insulin Human Isophane 100u/ml- $0.27, Insulin delivery device for Lily 3ml insulin cartridges (100units/ml) - $0.01 | Approved | |
| 6 | 3/1/2023 | SPC/Sri Lanka | Indent No: SPC/DM/454/2022 | Gabapentin capsules USP 300mg | Local Agent: Slim Pharmaceuticals Ltd Supplier/Manufacturer - Innova Captab, India | 8100 packs (10x10 capsules in a pack) | USD 0.02027 Per Capsule (LKR 7.40) | Permitted only for this consignment. No registered product suppliers have quoted. Price is below gazetted MRP. Permitted. |
| 7 | 3/1/2023 | SPC/Sri Lanka | Tender No: DHS/RP/307/2021 Proforma Invoice: PI-EXP-104-01-22 | Pazopanib Tablets 200mg | Supplier: TMI Solution (Pvt) Ltd Manufacturer: Hetero Healthcare Limited, India | 25,000 Tablets | LKR 330.00/ Tablet | Both manufacturer & local agent are not registered with NMRA. Purchase from registered sources. Not permitted. |
| 8 | 3/1/2023 | SPC/Sri Lanka | Tender No: DHS/P/WW/369/22 Proforma Invoice No: SHR/SL/INL/002-SEA | Hydroxocobalamin Injection USP 1mg/ml | Supplier:Innogen Lanka (Pvt) Ltd Manufacturer- Shrooq Pharmaceuticals Pvt Ltd, Pakistan | 280,000 Ampoules | LKR 86.90 per ampoule | No registered product MAH has quoted. Both Manufacturer & local agent are registered with NMRA. Only for this consignment. Permitted. |
| 9 | 3/1/2023 | SPC/Sri Lanka | Tender No: DHS/RP/ 290/21 Proforma Invoice No: CIR/EXD/22-23/602 | Ketamine Hydrochloride Injection USP 10mg/ml | Local Agent: Ceyoka Pvt Ltd Supplier/Manufacturer: Ciron Drugs & Pharmaceuticals Pvt Ltd, India | 18,000 vials | USD 2.20 per vial (LKR 803.54) | Permitted as manufacturer/local agent/higher strength are registered. Permitted |
| 10 | 3/1/2023 | SPC/Sri Lanka | Tender No: DHS/RP/141/2022 Proforma Invoice No: PI-EXP-150-01-23 | Pazopanib Tablets 200mg | Manufacturer -Hetero Healthcare Limited, India | 50,000 Tablets | LKR 330.00/ Tablet | Both manufacturer & local agent not registered. Not permitted. |
| 11 | 9/1/2023 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: AIL-SHL-thyro-01-2022-23 | Thyroxine Sodium Tablets IP 25 mcg (Thyronorm 25) | Manufacturer: Acme Generics (Pvt) Ltd, India | 150 000 Packs (30's) | USD 0.0187 Per Tablet (LKR 6.83) | Expedite registration approval. Not permitted. |
| 12 | 9/1/2023 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: AIL-SHL-thyro-01-2022-23 | Thyroxine Sodium Tablets IP 50 mcg (Thyronorm 50) | Manufacturer: Acme Generics (Pvt) Ltd, India | 150 000 Packs (30's) | USD 0.0233 Per Tablet | Expedite registration approval. Not permitted. |
| 13 | 9/1/2023 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: AIL-SHL-thyro-01-2022-23 | Thyroxine Sodium Tablets IP 100 mcg (Thyronorm 100) | Manufacturer: Acme Generics (Pvt) Ltd, India | 150 000 Packs (30's) | USD 0.04 Per Tablet (LKR 14.61) | Expedite registration approval. Not permitted. |
| 14 | 10/1/2023 | SPC/Sri Lanka | Indent No: SPC/CR/052/2021, SPC/CR/237/2022 & SPC/CR/332/2022 | Calcium Lactate Tablets 300 mg | Supplier/Manufacturer: Agog Pharma Ltd, India | 18 000 Packs + 48 000 Packs + 27 000 Packs | Calcium Lactate is not approved by MEC. Not permitted. | |
| 15 | 13/01/2023 | Niix Holdings (Pvt) Ltd | Proforma Invoice No: KE-2391 | Atracurium Besilate 10 mg/mL Solution for Injection/Infusion (25 mg in 2.5 mL Ampoule) | Manufacturer: AS 'Kalceks', Latvia | 2 000 Packs (5's) | EUR 1.192 Per Ampoule (LKR 465.93) | EU registered product Permitted as there is a shortage in the private sector. Permitted. |
| 16 | 13/01/2023 | Emar Pharma (Pvt) Ltd | Tender No: RES/11/04/A/2022 Indent No: SPC/DM/242/2022 | Gliclazide Modified Release Tablets 30 mg (GLIX MR 30) | Manufacturer: Wallace Laboratories (Pvt) Ltd, India | 66,000 Packs (10x10's) | USD 0.0210 Per Tablet (LKR 7.67) | Expedite site change. Not permitted. |
| 17 | 16/01/2023 | SPC/Sri Lanka | Proforma Invoice No: SB/22-23/PFI-39 Indent No:LP/DHS/EP/SA/3603/22 | Ceftriaxone for injection USP 1g | Supplier: Medica Pharmaceuticals (Pvt) Ltd Manufacturer: Schwitz Biotech, India | 500,000 vials | LKR 135.00/Vial | Both manufacturer & local agent not registered with NMRA. Purchase from registered manufacturer & local agent.Not permitted |
| 18 | 17/01/2023 | SPC/Sri Lanka | Proforma Invoice No: PA/PI/SPC/0009/22-23 PO No: 134330 Indent No: LP/DHS/EP/NV/3597/2022 Tender No: DHS/RP/EP/202/2022 | Docetaxel Injection 80 mg/2 mL | Supplier: Pharma Associates Manufacturer: SP Accure Labs Pvt Ltd, India | 4 000 Vials | LKR 2535.5100 Per Vial | Manufacturer is not registered with NMRA. Permiited for this consignment because of serious shortage & limited number of registered products. EU GMP for manufacturing site is available. Approved. |
| 19 | 17/01/2023 | Omacx Healthcare (Pvt) Ltd | Proforma Invoice No: SLI/22-23/53 & SLI/22-23/54 | Cefoperazone & Sulbactum for Injection USP 1 000 mg (CEFSULASH 1 000) | Manufacturer: Shamshree Lifesciences Ltd, India | 30 000 Vials & 30 000 Vials | USD 0.40 Per Vial (LKR 146.10) | Permitted as it is a registered product. Registration has lapsed. Should extend registration for future. Permitted only for this consignment. Permitted. |
| 20 | 20/01/2023 | Tabrane Pharmaceuticals (Pvt) Ltd | PO No: 132856 PA Order No: ICL/EOI/P1/80/2022 | Cytarabine Injection BP 1 000 mg /10 ml | Local Agent: Tabrane Pharmaceuticals (Pvt) Ltd Manufacturer: Naprod Life Sciences Pvt Ltd, India | 1 500 Vials | USD 5.7000 Per Vial (LKR 2081.90) | Permitted as the manufacturer/ local agent/ different strength is registered. Price is acceptable.Permitted. |
| 21 | 20/01/2023 | SPC Sri Lanka | PO No: 134427 Indent No: LP/DHS/EP/SA/3599/22 Tender No: DHS/RP/EP/187/2022 | Trastuzumab Powder for Solution for Infusion 440 mg (HERTICAD) | Supplier: Advitec International (Pvt) Ltd Manufacturer: Biocad Closed Joint Stock Company, Russia | 3 000 Vials | LKR 63450.0000 Per Vial | Purchase from registered products. Not permitted. |
| 22 | 24/01/2023 | Trimed Pharma (Pvt) Ltd | Proforma Invoice No: SL/002/2022 PO No: 129072 PA Order No: ICL/EOI/P1/184/2022 | Cyclophosphamide Injection IP 1 000 mg (Cycloxan) | Manufacturer: Cadila Healthcare Limited, India | 3 000 Vials | USD 3.7500 Per Vial (LKR 1369.67) | Previously registered product. Permitted. |
| 23 | 24/01/2023 | SPC Sri Lanka | Proforma Invoice No: UPF22054 PO No: 134901 Indent No: DHS/DA/483/2022 Tender No: DHS/RP/295/22 | Nilotinib Capsules 200 mg (Tasnib 200) | Supplier: Eureka Life Sciences Ptd Ltd, Singapore Manufacturer: Bruck Pharma (Pvt) Ltd, India Local agent: Yaden International (Pvt) Ltd | 15 000 Capsules (2x14's) | USD 2.56 Per Capsule (LKR 935.03) | Both manufacturer & local agent are registered. Permitted due to shortage. Permitted |
| 24 | 24/01/2023 | SPC Sri Lanka | Proforma Invoice No: UBPL/GEX/2223/0225 PO No: 136384 Indent No: DHS/TN/494/2022 Tender No: DHS/RP/265/2022 | Dactinomycin for Injection USP 500 mcg (DACTINOTEC 0.5) | Manufacturer & Supplier: United Biotech (Pvt) Ltd, India Local Agent: George Steuart Health (Pvt) Ltd | 400 Vials | USD 30.0000 Per Vial (LKR 10957.37) | Manufacturer & local agent registered. Permitted due to shortage. Permitted. |
| 25 | 24/01/2023 | SPC Sri Lanka | Proforma Invoice No: TABRANE-01/2023 PO No: 134876 Indent No: LP/DHS/DA/3627/2022 Tender No: DHS/RP/297/2022 | Nilotinib Capsules 150 mg (Nilonib-150) | Supplier: Tabrane Pharmaceuticals (Pvt) Ltd, Sri Lanka Manufacturer: Drug International Ltd, Bangladesh | 21 000 Capsules (30's) | LKR 897.0000 Per Capsule | Permitted as both manufacturer/local agent registered. Permitted. |
| 26 | 24/01/2023 | SPC Sri Lanka | PO No: 134654 Indent No: DHS/UD/481/2022 Tender No:DHS/RP/282/2022 Performa invoice: UBPL/GEX/2223/0220 | Daunorubicin Injection IP 20mg | Supplier/Manufacturer: United Biotech Pvt Ltd, India | 1500 vials | USD 5.00 per vial (LKR 1826.23) | Permitted as both manufacturer & local agent are registered. Permitted. |
| 27 | 24/01/2023 | SPC Sri Lanka | PO No: 134658 Tender No: DHS/RP/272/2022 Indent No: DHS/UD/480/2022 Performa Invoice: UBPL/GEX/2223/0222 | Vinblastine Sulphate Injection 10mg/10ml | Supplier/Manufacturer: United Biotech Pvt Ltd, India | 500 vials | USD 4.00 per vial (LKR 1460.98) | Registered product but not renewed. Shortage at MSD. Permitted |
| 28 | 24/01/2023 | SPC Sri Lanka | Tender No: DHS/RP/258/2022 Indent No: LP/DHS/MM/3633/2022 | Gemcitabine for Injection USP 200mg | Supplier/Local Agent: Softcare International (Pvt) Ltd Manufacturer: Jodas Expoim Pvt Ltd, India | 750 vials | LKR 1028.00 per vial | Registered product not renewed. Approved as there is a shortage. Permitted |
| 29 | 25/01/2023 | SPC Sri Lanka | Tender No: RES/ICL/01/2022 Indent No: SPC/NDB/103/2022 | Amoxicillin Capsules BP 250mg | Manufacturer: Micro Labs Ltd, India | 6,000,000 Capsules (60,000 packs - pack size:10x10's ) | USD 0.0161/ Capsule (LKR 5.88) | Permitted as local manufacture is inadequate. Permitted. |
| 30 | 27/01/2023 | Novachem Lanka Pvt Ltd | PA order No: ICL/EOI/P1/23/2022 PO No: 131103 Proforma Invoice No: PI 072/22-23 | Flupentixol Injection BP 20mg/ml | Manufacturer: Pharmafabricon, India | 11,250 Ampoules | USD 1.8 (LKR 657.44) | Previously registered. Price acceptable. Permitted. |
| 31 | 27/01/2023 | SPC Sri Lanka | Indent No: LP/DHS/EP/MMN/3620/2022 Tender No: DHS/RP/EP/199/2022 Proforma Invoice No: PA/PI/SPC/0008/22-23 | Imatinib Mesilate Capsules 400mg | Manufacturer: SP Accure Labs Pvt Ltd, India Supplier: Pharma Associates | 139,980 capsules/Tabets (2333 packs-pack size 60's) | LKR 59.6591 / Capsule | Permitted as there are no products in the country. Permitted. |
| 32 | 27/01/2023 | SPC Sri Lanka | PO No: 134443 Indent No: LP/DHS/EP/MMN/3623/2022 Tender No: DHS/RP/EP/200/2022 Proforma Invoice No: PA/PI/SPC/0008/22-23 | Imatinib Mesilate Capsules 400mg (SPATINIB 400) | Supplier: Pharma Associates Manufacturer: SP Accure Labs Pvt Ltd, India | 63,000 Tablets (30's) 2,100 Packs (Pack size: 30's) | LKR 127.841 (LKR 3835.23/ pack size 30's) | Permitted as there are no registered product in the market/state sector. Permitted. |
| 33 | 27/01/2023 | SPC Sri Lanka | Indent No: SPC/WM/472/2022 Tender No: RES/ICL/17/2022 | Ivabradine Hydrochloride Tablets 5mg | Manufacturer: Treffer Pharmaceuticals, India Supplier: Centurion Healthcare (Pvt) Ltd, India Local Agent: Yaden International (Pvt) Ltd | 12,000 packs ( pack size- 3x10's) | USD 0.045 / Tablet (LKR 16.44) [USD 1.35 / pack (3x10's)] | Manufacturer/ local agent/contract manufacturer registered with NMRA. Permitted due to short supply. Permitted |
| 34 | 1/2/2023 | SPC Sri Lanka | Indent No: LP/DHS/EP/PM/3604/2022 Proforma Invoice No:SR/22-23/PFI-40 Tender No: DHS/RP/EP/210/2022 | Furosemide Injection USP 20mg/2ml | Manufacturer- Schwitz Biotech, India | 625,000 Ampoules | USD 0.136 (LKR 49.67) [USD 1.36/ pack (10's)] | Both manufactuer & local agent not registered with NMRA. Purchase from registered supplier. Not permitted |
| 35 | 1/2/2023 | SPC Sri Lanka | PO No: 135223 Indent No: LP/DHS/MM/3634/2022 Tender No: DHS/RP/256/2022 Proforma Invoice No: PI014SS | Propofol 1% Injection for Intravenous infusion | Supplier/Local Agent: Unicura International (Pvt) Ltd Manufacturer: Kwality Pharmaceuticals Ltd, India | 12,500 Ampoules (1's) | LKR 774.20 per ampoule | Kwality has had several quality issues. NMRA has requested GMP inspection. Not Permitted. |
| 36 | 1/2/2023 | SPC Sri Lanka | Tender No: DHS/RP/151/2022 | Ifosamide for Injection USP 1gm with Mesna Injection 200mg/2ml | Local Agent: Imperial Life Sciences (Pvt) Ltd Manufacturer: Health Biotech Ltd, India | 800 Sets (03 amps per set) | USD 8.85 Per Set (LKR 3232.42) | Both manufacturer & local agent not registered with NMRA. MSD has 5 months stock. Not permitted. |
| 37 | 1/2/2023 | SPC Sri Lanka | Invoice No: EX21220234/AC Tender No:DHS/RP/148/2022 | Fluconazole Injection 200mg in 100ml | Supplier: SSK Pharma (Pvt) Ltd, Sri Lanka Manufacturer: Captab Biotech, India | 47,000 vials (1's, 100 mL) | USD 0.295 Per Vial (LKR 107.75) | Permitted as manufacturer is registered & price is acceptable. Permitted. |
| 38 | 1/2/2023 | SPC/Sri Lanka | PO No: 131514 Indent No: DHS/ICL/PM/469/2022 Tender No: DHS/RP/ICL/031/2022 Proforma Invoice No: PFI22230 | Brimonidine Tartrate Eye Drops 0.15% w/v | Supplier: Centurion Healthcare Private Limited, India Local Agent: Yaden International Mercury Laboratories Ltd, India | 43 000 Vials (1's) | USD 2.2000 Per Vial (LKR 803.54) | Not approved s eye surgeons have said non essential. Not Permitted. |
| 39 | 1/2/2023 | SPC/Sri Lanka | PO No: 135084 Indent No: DHS/PS/532/2022 Tender No: DHS/RP/263/2022 Proforma Invoice No: SRL 12 | Cyclophosphamide for Injection USP 200 mg (CTX-GLS 200) | GLS Pharma Ltd, India | 7 500 Vials (1's) | USD 0.3300 Per Vial (LKR 120.53) | Both manufacturer & local agent not registered. 1 gram vial was permitted. Not permitted. |
| 40 | 1/2/2023 | SPC/Sri Lanka | Tender No: DHS/RP/313/2021 Proforma Invoice No: E-PFI/963-TP-SL/22-23 | Ondansetron Injection 8 mg (Vomfran) | Supplier/ Manufacturer: Swiss Pharmaceuticals (Pvt) Ltd, Pakistan Local Agent: Talex Pharma (Pvt) Ltd | 200 000 Ampoules (10's) | USD 0.69 Per Ampoule (LKR 252.02) | Manufacturer is registered. Permitted as there is a shortage. Permitted. |
| 41 | 1/2/2023 | SPC/Sri Lanka | Tender No: DHS/RP/62/2022 Proforma Invoice No: UPF22044 | Sodium Acid Phosphate Effervescent Tablets | Supplier: Eureka Life Sciences Pte, Singapore Manufacturer: Vovantis Laboratories (Pvt) Ltd, India Local Agent: Yaden International | 100 000 Tablets (10's) | USD 1.65 Per Tablet (LKR 602.66) | Both manufacturer & local agent not registered. Also in non-essential list. Not permitted |
| 42 | 1/2/2023 | SPC/Sri Lanka | Tender No: RES/ICL/16/2022 Indent No: SPC/AD/446/2022 | Pantoprazole for IV Injection 40 mg (PANTODAC IV) | Manufacturer: Sakar Healthcare Limited,India | 48 000 Vials (1's) | USD 0.70 Per Vial (LKR 255.67) | Registered product, site change has taken place. Permitted. |
| 43 | 6/2/2023 | SPC/Sri Lanka | PO No: 137246 Indent No: LP/DHS/EP/MM/3649/2022 Tender No: DHS/RP/EP/304/2022 Proforma Invoice No: PA/PI/SPC/016/22-23 | Propofol Injection Emulsion USP 500 mg/50 mL (PROPO SPAL 500) | Supplier/Local Agent: Pharma Associates Manufacturer: SP Accure Labs Private Limited, India | 25 000 Ampoules (1's) | LKR 1290.0000 Per Ampoule | Manufacturer not registered. Product has a narrow therapeutic index. Not permitted. |
| 44 | 6/2/2023 | SPC/Sri Lanka | PO No: 134506 Indent No: LP/DHS/EP/MM/3616/2022 Tender No: DHS/RP/EP/197/2022 Proforma Invoice No: 157 | Gemcitabine Injection USP 200 mg/Vial (As lyophilized) (GEMDN 200) | Supplier/Local Agent: Yaden International (Pvt) Ltd Manufacturer: Biozenta (Pvt) Ltd, India | 750 Vials (1's) | 4,900.00 LKR/Vial | Manufacturer not registered. A registered product with lapsed registration was permitted today. Not permitted. |
| 45 | 6/2/2023 | SPC/Sri Lanka | PO No: 129762 Indent No: DHS/ICL/MM/455/2022 Tender No: DHS/ICL/X/011/2022 Proforma Invoice No: ZL/PI/16/2022 | Fenofibrate Capsules 200 mg (Fibrazee-200) | Supplier/ Manufacturer: Zee Laboratories (Pvt) Ltd, India Local Agent: ABC Pharma Services (Pvt) Ltd | 10 000 Packs (10x10's) | USD 1.6000 Per Capsule (LKR 584.39) | Both manufacturer & LA registered. Permitted as there is shortage. Permitted. |
| 46 | 7/2/2023 | A.Baur & Co. (Pvt) Ltd | Proforma Invoice No: 1301 | Glimepiride Tablets IP 3 mg (AMARYL 3) | Manufacturer: Sanofi India Limited, Verna-Goa, India Local Agent: A.Baur & Co. (Pvt) Ltd | 1600 Packs (2x30's) | 0.0608 Per Tablet | Expedite site change. Not Permitted. |
| Serial Number | Date of Submission | Local Agent | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Price quoted | CEO Decision |
| 1 | 8/11/2022 | ABC Pharma Services Pvt Ltd-Colombo 14 | DHS/ICL/MMN/449/2022 | Sodium Valproate Prolonged-Release Tablets 300mg | Manufacturer/Supplier - Zee Laboratories, India | 750,000 Tablets | CIF/USD 20.00 (1000 Tablets) | Approved |
| 2 | 8/11/2022 | ABC Pharma Services Pvt Ltd-Colombo 15 | DHS/ICL/MMN/450/2022 | Betahistine Tablets IP 8mg | Manufacturer/Supplier - Zee Laboratories, India | 3,000,000 Tabets | CIF/USD 3.50(per tablet of 1000) | Approved |
| 3 | 11/11/2022 | Junaht International Pvt Ltd- Mahabage | DHS/ICL/X/001/2022 | Magnesium Sulphate Inj. 50% in 10ml | Manufacturer - Coax Bioremedies Pvt Ltd, India Supplier - Infugen Pharma Pvt Ltd, India | 78,000 Ampoules | USD/CIF 0.17/ Ampoule | Approved |
| 4 | 2/1/2023 | TMI Solutions (Pvt) Ltd | ICL/EOI/P1/160/2022 | Enoxaparin Injection 40mg in 0.4ml | Manufacturer - Stanex Drugs & Chemicals Ltd, India Supplier - Questus Pharma Pvt Ltd, India | 200,000 PFSY | 1.8711 USD/PFSY | Approved(74 the MEC has approved maufacturer as Questus Pharma Pvt Ltd. LA has claimed manufacuturer is Stanex Drugs & Chemicals Ltd, India) |
| 5 | 2/1/2023 | TMI Solutions (Pvt) Ltd | ICL/EOI/P1/170/2022 | Enoxaparin Injection 60mg in 0.6ml | Manufacturer - Stanex Drugs & Chemicals Ltd, India Supplier - Questus Pharma Pvt Ltd, India | 249,999 PFSY | 2.2900 USD/PFSY | Approved(74 the MEC has approved maufacturer as Questus Pharma Pvt Ltd. LA has claimed manufacuturer is Stanex Drugs & Chemicals Ltd, India) |
| 6 | 11/1/2023 | Slim Pharmaceuticals (Pvt) Ltd | LP/DHS/EP/PM/3594/2022 | Meropenem for Injection USP 1g | Manufacturer - Gufic Biosicneces (Pvt) Ltd, India | 450,000 Vials | 1,895.50 LKR / Vial | Approved |
| 7 | 20-01-2023 | Slim Pharmaceuticals (Pvt) Ltd | LP/DHS/EP/WD/3591/22 | Dobutamine Injection 250mg/20ml | Manufacturer - Divine Laboratories (pvt) Ltd, India | 27,500 Vials | 1,140.00 LKR /Vial | Approved |
| 8 | 20-01-2023 | Slim Pharmaceuticals (Pvt) Ltd | LP/DHS/EP/PS/3598/2022 | Liposomal Amphotericin B Injection 50mg | Manufacturer - Gufic Biosicneces (Pvt) Ltd, India | 6,250 Vials | 37,250.00 LKR/Vial | Approved |
| 9 | 7/2/2023 | Unicura International (Pvt) Ltd | ICL/EOI/P2/11/2022 | Carvedilol Tablets BP 6.25mg | Manufacturer - Flourish Pharma, India | 62,50,000 Tablets | 0.0290 USD/Pack of 1000's | Approved |
| 10 | 1/2/2023 | Cliniqon Biotech Pvt. Ltd | DHS/RP/EP/208/2022 | Somatropin for Injection 2IU-30IU | Manufacturer - Cinnagen CO., Iran | 75,000 IU (2500 PACKS X 30) | 403.33 LKR/Vial | Approved |
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 30/11/2022 | Klintas (Pvt) Ltd | Proforma Invoice No: UBPL/GEX/2122/365 | Aciclovir Sodium for Infusion BP 250 mg (UNIVIR 250) | United Biotech (P) Limited, India | 2 000 Vials (1's) | USD 2.500 Per Vial (CIF Price) | Expidite registration. Not permitted. |
| 2 | 2/12/2022 | SPC/Sri Lanka | Proforma Invoice No: SP/E/0130 Tender No: DHS/P/WW/471/20 | Calcium Acetate Tablets USP 1000mg | Solitaire Phaamacia Pvt Ltd, India | 120 Boxes (Pack size 10x10) | USD 0.391 Per Tablet | Expedite registration. Not cost-effective when compared with Calcium Carbonate which is LKR 9/- Not pertmitted. |
| 3 | 5/12/2022 | Epidemiology Unit, SPC | Proforma Invoice No: 0000000001 | Bivalent Poliomyelitis Vaccine Type 1 & Type 3 Live (Oral) | Panacea Biotech Limited, India | 35 000 Vials (1's) | USD 2.45 Per Vial | Permitted as per the letter issued by Dr. Samitha Ginige, Acting Chief Epidemiologist, MOH. Permitted. |
| 4 | 8/12/2022 | SPC Sri Lanka | Proforma Invoice No: 0000000001 PO No: 131905 Indent No: DHS/TEP/085/22 Tender No: N/A | Bivalent Polio Vaccine 20 doses | Manufacturer: Panacea Biotech Limited, India Supplier: United Nations Children's Fund, Denmark | 35 000 Vials (1's) | USD 3.3000 Per Vial (CIF Price) & USD 2.45 Per Vial (FCA Value) | Permitted as per the letter issued by Dr. Samitha Gininge, Acting Chief Epidemiologist, MOH. Permitted. (Same as the 03rd application) |
| 5 | 12/12/2022 | SPC Sri Lanka | Proforma Invoice No: PI-Exp-104-11-22 Tender No: DHS/RP/305/2021 | Hydrocortisone Tablets USP 5 mg | Manufacturer: Jackson Laboratories (Pvt) Ltd, India Supplier: TMI Solutions (Pvt) Ltd | 225 000 Tablets (100's) | LKR 21.84 Per Tablet | Both local agent and manufacturer are not registered with NMRA. Recommend recall of tender. Not permitted. |
| 6 | 13/12/2022 | Breathe Free Lanka (Pvt) Ltd | Proforma Invoice No: BFL/22/248 | Formoterol Fumarate Dihydrate 6 mcg and Budesonide 100 mcg Inhaler | Cipla Ltd (Goa Site), India | 2 000 Packs (1's, 120 metered doses) | USD 2.68 Per Pack | Site change. But registered product. Permitted. |
| 7 | 13/12/2022 | Klintas Pvt Ltd | Invoice No: UBPL/GEX/2223/0254 | Protamine Sulphate Injection USP 50mg/50ml | United Biotech Pvt Limited, India | 1000 units | USD 5.50/unit | MRP of registered product is LKR 825/-. Not permitted. |
| 8 | 13/12/2022 | Siba Holdings | Invoice No: PI/0022/2022-23 | Gabapentin Capsules USP 100mg | Stallion Laboratories Pvt Ltd, India | 15,000 packs | USD 0.96/ Unit | Registered products available at MRP less than LKR 39.61. Not permitted. |
| 9 | 13/12/2022 | National STD/AIDS Control programme | Invoice No: MH2712104634 | Sofosbuvir/Velpatasvir Tablets 400mg/100mg | Mylan Laboratories, India | 265 Packs | USD 69.96/Pack | Global Fund. Permitted. |
| 10 | 14/12/2022 | SPC Sri Lanka (Local Agent: Mansel (Ceylon) Limited) | Proforma Invoice No: HLL-SLT1-12-12-2022 Tender No: DHS/P/WW/305/2022 | Dolutegravir Tablets 50 mg | Hetero Labs Ltd, India | 163 000 Tablets (30's) | USD 0.177 Per Tablet | Permitted but expedite registration. Permitted. |
| 11 | 14/12/2022 | SPC Sri Lanka | Tender No: DHS/RP/491/2020 | Oseltamivir Phosphate Capsules USP 75 mg (OSEVIR-FLU 75) | Sands Active (Pvt) Ltd, Sri Lanka | 50 000 Capsules (10's) | . | Registered product. Not permitted. |
| 12 | 14/12/2022 | SPC Sri Lanka (Local Agent: Yaden International (Pvt) Ltd) | Tender No: DHS/RP/491/2020 | Oseltamivir Capsules IP 75 mg (MCOSVIR) | Manufacturer: Mcneil & Argus Pharmaceuticals Limited, India Supplier: Eureka Life Science, Singapore | 50 000 Capsules (10's) | USD 0.58 Per capsule | At the time of calling tenders only the innovator has been registered. Innovator cost is high. Hence permitted only for this proceurement as several products have been registered on 2022. Permitted. |
| 13 | 15/12/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/RP/160/2022 | Cefotaxime for Injection USP 500 mg (CEFTAX 500) | Opsonin Pharma Ltd, Bangladesh | 12 500 Vials (1's) | USD 1.28 Per Vial (C&F Price) | Gazetted Price = 506.76 Permitted as 1 g is registered from same manufacturer. Request manufacturer to register 500mg. Permitted. |
| 14 | 16/12/2022 | Breathe Free Lanka (Pvt) Ltd | Proforma Invoice No: BFL/22/255 | Warfarin Sodium Tablets USP 1 mg (WARF-1) | Cipla Ltd (Malpur Site), India | 70 000 Packs (10x10's) | USD 1.42 Per Tablet | Permitted due to shortage arising. Site change of a registered product. Permitted. |
| 15 | 16/12/2022 | Breathe Free Lanka (Pvt) Ltd | Proforma Invoice No: BFL/22/243 | Solifenacin Succinate Tablets IP 5 mg (SOLIACT 5) | Cipla Ltd (Sikkim Site), India | 15 000 Packs (1x15's) | USD 2.75 Per Tablet | Permitted as it is a site change of a registered product. Price is within MRP. Permitted. |
| 16 | 16/12/2022 | Emar Pharma (Pvt) Ltd | Proforma Invoice No: WPPL/39/2022-23 Tender No: RES/ICL/08/2022 Indent No: SPC/DM/259/2022 | Gliclazide Modified Release Tablets 30 mg (GLIX MR 30) | Wallace Laboratories (Pvt) Ltd, India | 66 000 Packs (10x10's) | USD 0.0210 Per Tablet (C&F Price) | Previously registered product. Site change has taken place. Permitted. |
| 17 | 20/12/2022 | SPC Sri Lanka | Tender No: DHS/RP/472/2020 | Phytomenadione Tablets BP 5 mg | Manufacturer: Mercury Laboratories Ltd, India Supplier: Eureka Life Sciences Pte Ltd, Singapore | 49 000 Tablets (100's) | Not mentioned | Permitted as there is a acute shortage. Should register for future. Permitted. |
| 18 | 20/12/2022 | SPC Sri Lanka | Tender No: RES/ICL/13/2022 Indent No: SPC/PG/470/2022 | Betamethasone Dipropionate 0.05% + Gentamycin Sulphate Cream | Manufacturer: Belco Pharma, India Supplier: Pharmatech | 5000 Packs (1's, 15 g) | USD 0.855 Per Pack (C&F Price) | Topical product with Gentamicin are not permitted by NMRA. Not permitted. |
| 19 | 22/12/2022 | SPC | Tender No. DHS/AMS/460/22 | Yellow Fever Vaccine 0.5ml Single Dose | Manufacturer : Sanofi Pasteur , France Supplier: Sanofi Healthcare India (Pvt) Ltd, India | 1,500 Ampoules | - | Permitted as there is no vaccine in the country. Permitted. |
| Appeals & Requests of Extensions for the Already Issued WORs, Other | ||||||||
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 2/12/2022 | SPC Sri Lanka | PO NO: 84801 Indent No: DHS/WD/140/21 Tender No: DHS/P/WW/684/21 | Linezolid Tablets 600 mg (LIZOLID 600) | Manufacturer: Glenmark Pharmaceuticals Ltd, India Local Agent: Sunshine Healthcare Lanka Ltd | 30 000 Tablets (5x1x4's) | USD 7.6900 Per Tablet (C&f Price) | Permitted as it is an extension of of previous WOR. Permitted. |
| 2 | 15/12/2022 | SPC Sri Lanka | PO No: 111226 Tender No: DHS/RP/193/2021Indent No: DHS/AR/469/2021 | Cefuroxime Sodium Injection BP 250 mg | Neon Laboratories Ltd, India | 100 000 Vials (1's) | USD 1.3000 Per Vial | Permitted for this consignment only. Should register product. Permitted. |
| Serial Number | Date of Submission | Applicant | Quotation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 12/10/2022 | SPC/Sri Lanka | Tender No: DHS/RP/481/2020 Proforma Invoice No: FBIPL-22-23/082 | Chloramphenicol Sodium Succinate for Injection BP 500 mg | Divine Laboratories (Pvt) Ltd, India | 5 000 Vials | USD 5.00 Per Vial | To check the utilization as the price is high. Decision Pending |
| 2 | 17/10/2022 | A.Baur & Co. (Pvt) Ltd | Proforma Invoice No: 1294 | Clobazam Tablets IP 10 mg (FRISIUM) | Sanofi India Ltd (Goa Site), india | 5 146 Packs (450's) | USD 21.83 Per Pack | Expedite the registration. Not permitted. |
| 3 | 17/10/2022 | A.Baur & Co. (Pvt) Ltd | Proforma Invoice No: 1285 | Glimepiride Tablets IP 2 mg (AMARYL 2) | Sanofi India Ltd (Goa Site), india | 12 000 Packs (60's) | USD 2.87 Per Pack | Expedite the registration. Not permitted. |
| 4 | 17/10/2022 | Sunshine Healthcare Lanka Ltd | Indent No: SPC/BS/059/2022 | Vitamins and Minerals Supplement (VISAVIT) | Remington Pharmaceuticals Industries (Pvt) Ltd, Pakistan | 54 000 Packs (30's) | USD 0.0273 Per Tablet | Await Borderline Decision . Not permitted. |
| 5 | 15/11/2022 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: TMPL/PI/2022-23/31 | Esomeprazole Tablets 40 mg (ESOPEL 40) | Medopharm (Pvt) Ltd, India | 2 000 Packs (10x10's) | USD 0.1076 Per Tablet (CIF Price) | Expedite the registration. Not permitted. |
| 6 | 15/11/2022 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: TMPL/PI/2022-23/31 | Clopidogrel Tablets USP 75 mg (MEDOVIX) | Medopharm (Pvt) Ltd, India | 8 333 Packs (3x10's) | USD 0.0503 Per Tablet (CIF Price) | Expedite the registration. Not permitted. |
| 7 | 17/10/2022 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: TMPL/PI/2022-23/31 | Loratadine Tablets USP 10 mg (LORAHIST) | Medopharm (Pvt) Ltd, India | 5 000 Packs (10x10's) | USD 0.0406 Per Tablet (CIF Price) | Expedite the registration. Not permitted. |
| 8 | 21/10/2022 | SPC/Sri Lanka | Proforma Invoice No: SP/E/0041 PO No: 59051 Indent No: LP/DHS/HD/2822/2020 Tender No: DHS/P/WW/255/20 | Cholecalciferol Tablets BP 5000 IU | Supplier: Orexo Bio Pharma Pvt Ltd Manufacturer: Solitaire Pharmaceuticals India | 1 000 Packs (10x10's) | LKR 40.00Per Tablet | Not approved.Both manufacturer and supplier not registered with NMRA. |
| 9 | 28/10/2022 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: 90006805 Tender No: RES/08/04/C/2020 Indent No: SPC/AD/257/2020 | Cefuroxime Axetil Tablets USP 250 mg (ALTACEF 250) | Zeiss Pharma Ltd, India | 24 000 Packs (5x10's) | USD 0.1012 Per Tablet | SPC indicated that this is an ICL order. Hence refered to ICL pathway. Not permitted. |
| 10 | 8/11/2022 | Sunshine Healthcare Lanka Ltd | PO No: 84801 Tender No: DHS/P/WW/684/21 Indent No: DHS/WD/140/21 Proforma Invoice No: EXP/0022/22-23 | Linezolid Tablets 600 mg (LIZOLID-600) | Glenmark Pharmaceuticals Ltd, India | 1 500 Packs (1x4x5's) | USD 7.6900 Per Tablet (C&F Price) | Already registered. Not permitted. |
| 11 | 8/11/2022 | Niix Holdings (Pvt) Ltd | Proforma Invoice No: 90022108 | Heparin Solution for Injection 25 000 IU/5 mL | Galenika a.d. Beograd, Republic of Serbia for Mefar ILAC Sanayii A.S., Turkey | 1 000 Packs (10x5 mL) | EUR 3.51 Per Ampoule | This product is not centrally registered in EU. It has decentralized registration in Serbia. Product has not been used previously in SL. Consultant physician contacted the private hospitals & discussed the issue. Awaiting a response in writing regarding the ADRs. Shared the list of registered products with private hospitals. Decision Pending |
| 12 | 11/11/2022 | ABC Pharma Services (Pvt) Lt | Proforma Invoice No: 1.) 01/OCTA/2/2022-23 2.) 02/OCTA/2/2022-23 | Human Albumin Solution 20% w/v (ALBUNORM 20%) | Octapharma- AG, Switzerland | 1.) 17 651 (1's, 50 mL) 2.) 16 349 (1's, 50 mL) | USD 42.50 Per Vial (CIF Price) | Permitted as it is registered by stringent authority & labels are within the required information. |
| 13 | 15/11/2022 | Tabrane Pharmaceuticals (Pvt) Ltd | PO No: 125912 Indent No: LP/NP/DHS/B/E/014/2019 Tender No: DHS/RP/NP/B/007/2019 | Ledipasvir 90 mg + Sofosobusvir 400 mg Tablets (HOPSO-LP) | Drug International Ltd, Bangladesh | 84 Tablets (7's) | LKR 990.0000 Per Tablet | Permitted as no registered product & price is accepted after checking. |
| 14 | 16/11/2022 | ABC Pharma Services (Pvt) Lt | PO No: 129 075 Proforma Invoice No: 27/GL/EXP/2022-23 | Desferrioxamine Mesilate for Injection BP 500 mg | Gland Pharma Limited, India | 31 000 Vials (10's) | USD 5.10 (CIF Price) Per Vial | To register the product SPC informed that tender has been cancelled. Not permitted. |
| 15 | 17/11/2022 | SPC/Sri Lanka | PO No: 92937 Indent No: DHS/NV/334/2021 Tender No: DHS/P/WW/641/2021 | Docetaxel Concentrate for Injection USP 80 mg/2 mL (With Solvent) | Naprod Life Sciences (Pvt) Ltd, India | 19 000 Vials (1's) | USD 5.1500 Per Vial | Get more information. SPC has cancelled tender. Not permitted. |
| 16 | 17/11/2022 | SPC/Sri Lanka | Tender No: DHS/RP/217/2021 | Albendazole Oral Suspension USP 200 mg/ 5 mL | Lavita Pharma Pvt Ltd, India | 2 500 Bottles (30 mL) | LKR 972.00 Per Bottle | Both manufacturer and supplier not registered. Not permitted. |
| 17 | 22/11/2022 | Yaden International (Pvt) Ltd | PO No: 130 762 PA Order No- ICL/EOI/P1/59/2022 | Tetracosactrin Injection 250mcg | Mercury Laboratories Ltd, India | 500 Ampoules (1's, 1 mL) | LKR 120.00 Per Ampoule | MSD has not participated. Hence no dicision can be made. Have a new meeting with MSD included to make a dicision. Decision Pending |
| 18 | 22/11/2022 | Yaden International (Pvt) Ltd | PO No: 130 764 PA Order No- ICL/EOI/P1/67/2022 | Balanced Salt solution 500ml (EQUASOL) | Sunways India (Pvt) Ltd | 8750 Bottles (1's. 500 mL) | LKR 12.00 Per Bottle | MSD has not participated. Hence no dicision can be made. Have a new meeting with MSD included to make a dicision. Decision Pending |
| 19 | 22/11/2022 | Yaden International (Pvt) Ltd | PO No: 130 765 PA Order No- ICL/EOI/P1/106/2022 | Ergometrine Injection BP 500 mcg | Mercury Laboratories Ltd, India | 8751 Ampoules (1's, 1 mL) | LKR 2.00 Per Ampoule | MSD has not participated. Hence no dicision can be made. Have a new meeting with MSD included to make a dicision. Decision Pending |
| 20 | 22/11/2022 | Yaden International (Pvt) Ltd | PO No: 130 774 PA Order No- ICL/EOI/P1/187/2022 | Leucovorin Calcium Tablets USP 15 mg (LEUVOR 15) | Bruck Pharma (Pvt) Ltd, India | 6 000 Tablets (10's) | USD 2.5000 Per Tablet | MSD has not participated. Hence no dicision can be made. Have a new meeting with MSD included to make a dicision. Decision Pending |
| 21 | 22/11/2022 | Yaden International (Pvt) Ltd | PO No: 130 769 PA Order No-ICL/EOI/P1/133/2022 | Fusidic Acid Eye Drops 1% w/v | Mercury Laboratories Ltd, India | 37 500 Vials (1's, 10 mL) | USD 5.0000 Per Vial | MSD has not participated. Hence no dicision can be made. Have a new meeting with MSD included to make a dicision. Decision Pending |
| 22 | 22/11/2022 | Yaden International (Pvt) Ltd | PO No: 130 757 PA Order No-ICL/EOI/P154/2022 | Propylthiouracil Tablets BP 50 mg | Centurion Laboratories (Pvt) Ltd, India | 62 500 Tablets (60's) | USD 1.50000 Per Tablet | MSD has not participated. Hence no dicision can be made. Have a new meeting with MSD included to make a dicision. Decision Pending |
| 23 | 25/11/2022 | SPC/Sri Lanka | Tender No: DHS/P/WW/124/21 | Colchicine Tablets 500mcg (GOUTNIL) | Inga Laboratories Pvt Ltd, India | 110 000 Tablets (15x10's) | USD 0.0253 Per Tablet | Several times WOR committee has informed to expedite the registration. Ensure that the dossier is evaluated & inform WOR committee the reason for the long delay. Decision Pending |
| 24 | 25/11/2022 | SPC/Sri Lanka | Tender No: DHS/RP/438/2020 Proforma Invoice No: P/DEX/048/2022 | Methotrexate Injection USP 100 mg (CYTOTREX 100) | BDH Industries Ltd, India | 2 000 Vials (10 mL) | USD 8.55 Per Vial (C&F Price) | Permitted only for this procurement. Will not be considered unless registered at NMRA. |
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 2/9/2022 | Breathe Free Lanka Pvt Ltd | Invoice No: BFL/22/133 | Dutasteride soft gelatin capsules IP 0.5mg | Manufacturer: Cipla Ltd, India Local Agent : Breathe Free Lanka Pvt Ltd | 20,000 packs | USD 20.48/Pack (10x10's) | Permitted as Cipla ltd (parent company) is registerd in NMRA. (Due to the pandemic situation) |
| 2 | 077/09/2022 | Baur Life Sciences (Pvt) Ltd | Proforma invoice No :BPL/22-23:59 Indent No :4200007529 | Cephalexin Oral Suspension BP 125mg/5ml | Manufacturer: Bharat Parenterals Ltd, India | 25,000 packs | USD 0.810/unit | To expedite the registation |
| 3 | 2/9/2022 | Pharma associates | Proforma invoice: 004/AMN/SRL/22-23 Indent No: DHS/PM/85/21 | Teicoplanin for injection 400mg | Manufacturer: AMN LifeSciences Pvt Ltd, India | 20,000 vials | USD 3.97 | Several quality failures for the parenterals from this manufacturer. Currently no valid registation. Registration has expired in May 2020. GMP inspection has been requested by NMRA for further registrationsTherefore not permitted . |
| 4 | 7/9/2022 | Siba Holdings Pvt Ltd | Invoice No: PI/0191/2022-23 Indent No: SPC/CR/091/21 Tender :RES/04/03/C/2021 | Captopril Tablets BP 25mg | Manufacturer: Stallion Laboratories Pvt Ltd, India | 5,000 packs | USD 0.0081 | Coming through indian creditline. Hence permitted |
| 5 | 7/9/2022 | Emar Pharma Pvt Ltd | Tender No: RES/11/04/A/2022 | Gliclazide modified Release Tablets 30mg | Manufacturer: Wallace Laboratories Pvt Ltd, India | 66,000 packs | Expedite the evaluation. | |
| 6 | 9/9/2022 | Care Ring Pharma Pvt Ltd | Indent No: LP/DHS/PH/3484/2022 Tender : DHS/P/WW/325/2022 | Talc Powder Sterile 4g bottle | Manufacturer: Indeus Life Sciences Pvt Ltd, India | 500 packs | LKR 9,960.00 per BOT of 1 | Both local agent & manufacturer not registered with NMRA. Hence not permitted |
| 7 | 16/09/2022 | Hemas Pharmaceuticals (Pvt) Ltd | Proforma Invoice No: SUN/HEM/004/SEP/2022 | 1. Bimatoprost Eye Drops 0.03 w/v (CAREPROST) 2. Olopatadine Bimatoprost Eye Drops (WINOLAP DS) 3. Carboxy Methylcellulose Eye Drops (TEAR DROPS) 4. Brinzolamide Eye Drops 1% w/v (BRINOLAR) 5. Nepafenac Eye Drops 0.1% w/v (NEPALACT) 6. Timolol Eye Drops 0.5% w/v 7. Dexamethasone and Moxifloxacin Eye Drops (MILFLODEX BKC Free Sterile Eye Drops) | Manufacturer: Sun Pharmaceutical Industries Limited, Halol, India | 1.) 2 500 Boxes (1x1) 2.) 3 500 Boxes (1x1) 3.) 6 000 Boxes (1x1) 4.) 3 000 Boxes (5 mL) 5.) 2 000 Boxes (1x1) 6.) 1 000 Boxes (1x1) 7.) 4 500 (1x1) | 1.) USD 4.00 (CIF Price) 2.) USD 2.20 (CIF Price) 3.) USD 1.45 (CIF Price) 4.) USD 3.5 (CIF Price) 5.) USD 2.00 (CIF Price) 6.) USD 4.70 (CIF Price) 7.) USD 2.00 (CIF Price) | Inform Local agent to submit seperate applications for each products.Permitted as Sun Pharmaceutical Industires Ltd, India (parent company) is registerd in NMRA. (Due to pandemic situation) |
| 8 | 20/09/2022 | A. Baur & Co. (Pvt) Ltd | Proforma Invoice No: 1200011993 | Capecitabine Tablets 500 mg (XELODA) | Excella GmbH, Germany for F.Hoffmann-La Roche Limited, Switzeland | 100 EA | USD 245.22 Per EA | Permitted as Roche (parent company) is registerd in NMRA. |
| 9 | 22/09/2022 | Sukhee Pharma (Pvt) Ltd | Proforma Invoice No: 22225697 | Neostigmine Concentrate for Solution for Injection/Infusion 0.5 mg/mL (Neostigmine-hameln) | Siegfried Hameln Gmbh, Germany | 20 000 Ampoules | Eur 5.30 | Submit dossier for consideration of registration. Otherwise should provide evidance of shortage in private sector |
| 10 | 22/09/2022 | Sukhee Pharma (Pvt) Ltd | Proforma Invoice No: 22225705 | Amiodarone Hydrochloride Concentrate for Solution for Injection/Infusion 50 mg/mL | Siegfried Hameln Gmbh, Germany | 10 000 Ampoules | Eur 10.00 | Submit dossier for consideration of registration. Otherwise should provide evidance of shortage in private sector |
| 11 | 26/09/2022 | SMM Halcyon (Pvt) Ltd | PO No: 123011 Tender No: DHS/RP/NP/B/537/2021 Indent No: LP/NP/DHS/B/MMN/538/2021 | Tenofovir Alafenamide Tablets 25 mg (HEPBEST) | Mylan Laboratories, India | 360 Tablets (30's) | LKR 200.0000 Per Tablet | Permitted only for this procument. If further needed manufacturer has to register the product |
| 12 | 9/9/2022 | SPC/Sri Lanka | Tender No: DHS/ICL/X/007/2022 Invoice No: PFI22094 | Disulfiram Tablets BP 250 mg | Mercury Laboratories Limited, India | 200 000 Tablets | USD 0.30 Per Tablet (C&F Price) | Quality failures were reported to the same product and not an essential medicine. Not permitted |
| 13 | 28/09/2022 | Niix Holdings Pvt Ltd | Proforma Invoice - 90484322 | Magnesium Sulphate injection 50% 10 ml | Macarthys Laboratories Limited T/A Martindale Pharma, United Kingdom | 5000 Ampoules (500 packs) | USD 11 / Pack (10x10ml) | Registered in stringent authority hence can consider. But should provide evidance of shortage in the private sector |
| 14 | 4/10/2022 | SSK Pharma Pvt Ltd | Invoice No- SSK/DHS-RP/262 | Flucanazole Injection 200mg in 100ml vial | Manufacturer- Captab Biotech, India Supplier - SSK Pharma Pvt Ltd | 33,000 vials | LKR 195.00 | Manufacturer not registered. COPP issued to Yemen avaiable. Not permitted |
| 15 | 5/10/2022 | Cloud Healthcare (Pvt) Ltd | PO No: 118804 Indent No: DHS/ICL/MM/152/2022 Tender No: DHS/ICL/X/005/2022 Proforma Invoice No: ORB/I/P/22/020 | Lidocaine and Prilocaine Cream USP 5% w/w (ORPRILO) | Orbit Pharmaceuticals, India | 7 000 Tubes (5 g) | USD 0.6 Per Tube | Coming under indian credit line. Hence permitted |
| 16 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: TMPL/PI/2022-23/30 | Esomeprazole Tablets 20 mg (ESOPEL 20) | Medopharm (Pvt) Ltd, India | 3 750 Packs (10x10's) | USD 7.54 (CIF Price) | Expedite the evaluation | |
| Appeals & Requests of Extensions for the Already Issued WORs, Other | ||||||||
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 2/9/2022 | Tabrane Pharmaceuticals | Invoice No : TABRANE-15/2021 | Remdesivir Lyophilized powder for IV infusion (DILAVIR) | Manufacturer : Drug International Ltd (Unit-2), Bangladesh | 10,000 Vials | Previous decision by MEC not needed. Submit to MEC committee for decision | |
| 2 | 20/09/2022 | SPC Sri Lanka | Tender No: RES/ICL/12/2022 | Erythromycin Stearate Tablets BP 500 mg | Supplier & Manufacturer: Micro Labs Limited, India | 4,000 Packs of 10x10's Blister | USD 6.74 Per 10x10's Blister | Permitted |
| 3 | 4/10/2022 | SPC | Tender No - LP/DHS/NP/E/013/2019 | Olaparib Capsule 50mg | Manufacturer - Drugs International Ltd, Bangladesh Supplier - Tabrane Pharmaceutical Pvt Ltd | 18,080 Capsules | LKR 650.00/ Capsule | Expedite registration |
| 4 | 4/10/2022 | SPC/Sri Lanka | Tender No: RES/07/05/C/2021 Indent No: SPC/EF/064/21 Commercial Invoice No: 461220113 Proforma Invoice No: PFI21221135 | Pregabalin Capsules 150 mg | Manufacturer: Sai Mirra Innopharm (Pvt) Ltd, India Supplier: Indus Lifescience (Pvt) Ltd, India | 12 500 Packs (10x10's) | USD 2.17 Per 10x10's Blister | Permitted to extend |
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 20/07/2022 | SPC/Sri Lanka | Indent No: DHS/HD/390/2019 | Paclitaxel Injection USP 6 mg (Administration device set + Sodium Chloride 0.9% w/v 500 mL glass bottle for every six vials of Paclitaxel Injection USP 6 mg in 5 mL vial) | Supplier: Belco Pharma, India Manufacturer for Diluent: Albert David Ltd, India Manufacturer for Administration Device: Angiplast Pvt Ltd, India | 4 500 -Administration Device Sets 4 500-Sodium Chloride 0.9% w/v 500 mL Glass Bottle | Permission given for bottle & giving set. Product is registered with NMRA. Previously used in SL. Permitted. | |
| 2 | 25/07/2022 | SPC/Sri Lanka | Tender No: DHS/ICL/X/008/2022 | Sunitinib Malate Capsules 50 mg | Manufacturer: SP Accure Labs (Pvt) Ltd, India | 10 000 Capsules | USD 1.23 per Capsule | No registered supplier. Hence, permitted to the lowest price bidder Accure Labs (Pvt) Ltd, India. Permitted |
| 3 | 27/07/2022 | SPC/Sri Lanka | Indent No: DHS/SA/589/18 | Morphine Sulfate Oral Solution BP 0.2% w/v | Manufacturer: BDH Industries Ltd, India | 1 000 Bottles | Permitted as it is coming through Indian Credit Line & there are no registered product. Previously supplied by them with no quality failures. Permitted. | |
| 4 | 28/07/2022 | SPC/Sri Lanka | Tender No: RES/ICL/01/2022 Indent No: SPC/NDB/103/2022 | Amoxicillin Capsules Bp 250 mg | Supplier & Manufacturer: Micro Labs Limited, India | 60 000 Packs of 10x10's Blister | USD 1.6100 Per Pack | Buy from local manufacturer. Product not registered with NMRA. Not permitted. |
| 5 | 28/07/2022 | SPC/Sri Lanka | Tender No: RES/ICL/12/2022 | Erythromycin Stearate Tablets BP 500 mg | Supplier & Manufacturer: Micro Labs Limited, India | 4 000 Packs of 10x10's Blister | USD 674 Per Pack (C&F Price) | Permitted. |
| 6 | 29/07/2022 | SPC/Sri Lanka | PO No: 89375 Tender No: DHS/P/WW/250/21 Indent No: DHS/IG/303/21 | Prednisolone Acetate Ophthalmic Suspension USP 1.0% w/v (IO-PRED-S) | Supplier: Alvita Pharma (Pvt), India Manufacturer: Indiana Ophthalmics, India | 100 000 Vials | USD 4.4750 Per Vial | Expedite renewal of registration. Not permitted. |
| 7 | 29/07/2022 | Emerchemie NB (Ceylon) Limited | Tender No: DHS/P/WW/305/2022 | Dolutegravir Tablets 50 mg | Manufacturer: Macleods Pharmaceuticals Limited, India | 489 000 Tablets | USD 4.27 Per 30's (C&F Price) | Permitted as there are no registered products. Permitted. |
| 8 | 29/07/2022 | Niix Holdings (Pvt) Limited | Proforma Invoice No: KE-1809 | Noradrenaline (Norepinephrine) 1 mg/mL concentrate for Solution for Infusion | Manufacturer: Joint Stock Company 'Kalceks', Latvia | 10 000 Ampoules | EU registered medicine. It is a vital drug. Price is affordable & reasonable compared to previoulsy quoted prices from India. Permitted. | |
| 9 | 29/07/2022 | Junaht International Pvt. Ltd | Tender No: DHS/ICL/X/001/2022 Proforma Invoice No: 2022-23/ESO/ESU/00047 | Chloramphenicol Injection 500 mg | Supplier: Infugen Pharma Pvt Ltd, India Manufacturer: Jackson Laboratories Pvt Ltd, India | 5 000 Vials | USD 1.20 Per Vial (C&F Price) | Not permitted. |
| 10 | 29/07/2022 | CIC Holdings PLC | Invoice No: INI2233001604 | Plasma Lyte A Solution for Infusion 500 mL (Plasma Lyte A) | Bieffe Medital S.A., Spain | 3 500 Pcks | USD 1.3 Per Pack | Permitted as it is needed at LRH vide letters attached & it is a donation. |
| 11 | 8/8/2022 | Novachem Lanka (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 Proforma Invoice No: PROFORMA/10 | Gentamycin Eye Drops 0.3% w/v | Manufacturer: Modern Laboratories, India | 20 000 Vials | USD 0.114 Per Vial (C&F Air/Sea Price) | Permitted as it is coming through India Credit Line & LA is registered with NMRA. Permitted. |
| 12 | 8/8/2022 | Tabrane Pharmaceuticals (Pvt) Ltd | Proforma Invoice No: TABRANE-05/2022 | Remdesivir Lyophilized Powder 100 mg for IV Infusion (DILAVIR) | Manufacturer: Drug International (Unit 2), Bangladesh | 1 000 Vials | USD 8.50 (Sri Lanka FOB Price) | Advisory committee on Covid Medicine was of the opinion that it is not needed. Decision was made by the expert committee in 2021. Not permitted. |
| 13 | 8/8/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/002/2022 | Danazole Capsules USP 100 mg | Supplier: Centurion Healthcare Private Limited, India Manufacturer: Centurion Laboratories Private Limited, India | 100 000 Capsules | USD 0.44 Per Capsule (C&F Sea/Air Price) | No registered product. Permitted. |
| 14 | 10/8/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 | Chlorambucil Tablets BP 2 mg | Manufacturer: Bruck Pharma Private Limited, India | 30 000 Tablets | USD 1.84 Per Tablet (C&F Sea/Air Price) | Needed in Oncology. Permitted. |
| 15 | 10/8/2022 | Pharma Associates | PO No: 118807 Tender No: DHS/ICL/X/008/2022 Indent No: DHS/ICL/TEP/153/22 Proforma Invoice No: SPAL/22-23/EPI/010, SPAL/22-23/EPI/009 & SPAL/22-23/EPI/008 | Pazopanib Tablets 200 mg (PAZOSPAN 200) | Manufacturer: SP Accure Labs Pvt. Ltd., India | 54 000 Tablets | USD 3.0500 Per Tablet (C&F Price) | Permitted as no registered generics. Permitted. |
| 16 | 16/08/2022 | SPC/Sri Lanka | Tender No: RES/ICL/01/2022 Indent No: SPC/NDB/149/2022 | Amoxicillin Capsules Bp 250 mg | Manufacturer: Micro Labs Limited, India | 8 000 Packs (1000's Per HDPE Bottle Packs) | USD 12.80 Per 1000's Pack (C&F Price) | Permitted as local manufacturer is inadaquate. Permitted. |
| 17 | 16/08/2022 | Sunshine Healthcare Lanka Ltd | Tender No: RES/18/02/C/2021 Indent No: SPC/WM/071/2021 | Meloxicam Tablets BP 15 mg | Manufacturer: Cadila Pharmaceuticals Ltd, India | 31 500 Packs (10x10's Blister) | USD 1.34 Per 10x10's Pack (C&F Price) | Extend registration. Permitted. |
| 18 | 16/08/2022 | Pharmatec Private Limited | PO No: 117942 Tender No: DHS/ICL/X/002/2022 Indent No: DHS/ICL/TN/125/2022 | Testosterone Enanthate Injection USP 250 mg/mL | Manufacturer: Belco Pharma, India | 8 500 Ampoules (10's) | USD 19.8 Per Ampoules of 10's | Past supplier. Permitted |
| 19 | 16/08/2022 | Pharmatec Private Limited | PO No: 117778 Tender No: DHS/ICL/X/002/2022 Indent No: DHS/ICL/MM/122/2022 | Metoprolol Tartrate Injection 1 mg/mL | Manufacturer: Belco Pharma, India | 800 Ampoules (10's) | USD 52.2000 Per 10's | Permitted as it is from Indian Credit Line. Permitted. |
| 20 | 16/08/2022 | Pharmatec Private Limited | PO No: 117807 Tender No: DHS/ICL/X/002/2022 Indent No: DHS/ICL/MM/124/2022 | Testosterone Undecanoate Injection 250 mg/mL | Manufacturer: Belco Pharma, India | 1 000 Vials (10's) | USD 126.0000 Per Vial of 10) | Past supplier. Permitted. |
| 21 | 16/08/2022 | Junaht International Pvt. Ltd | Tender No: DHS/ICL/X/001/2022 Proforma Invoice No: 2022-23/ESO/ESU/00049 | Magnesium Sulphate Injection BP/USP 50% in 10 mL | Manufacturer: Coax Bioremedies Pvt. Ltd, India | 78 000 Ampoules | USD 0.17 Per Ampoule (C&F Sea/Air Price) | Decision Pending- Documents pending from SPC. |
| 22 | 16/08/2022 | Lanka Therapeutics (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 Proforma Invoice No: 004/22-23 | Ruxolitinib Tablets 20 mg (RUXOVEON 20) | Manufacturer: Aeon Formulations (Pvt) Ltd, India | 14 000 Tablets (2x14's) | USD 17.78 Per Tablet (C&F Air/Sea Price) | Not Permitted. Checked with College of Haematologists extremely rarely needed. |
| 23 | 16/08/2022 | Lanka Therapeutics (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 Proforma Invoice No: 005/22-23 | Ruxolitinib Tablets 5 mg (RUXOVEON 5) | Manufacturer: Aeon Formulations (Pvt) Ltd, India | 24 976 Tablets (2x14's) | USD 8.95 Per Tablet (C&F Air/Sea Price) | Not as essential medicine. Not Permitted |
| 24 | 16/08/2022 | Lanka Therapeutics (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 Proforma Invoice No: 006/22-23 | Ruxolitinib Tablets 15 mg (RUXOVEON 15) | Manufacturer: Aeon Formulations (Pvt) Ltd, India | 7 476 Tablets (2x14's) | USD 17.73 Per Tablet (C&F Air/Sea Price) | Decision made in consultation with College of Haematologists that it is a non-essential medicine. Not permitted. |
| 25 | 16/08/2022 | Siba Holdings (Pvt) Ltd | Tender No: RES/26/11/B/2021 Indent No: SPC/CR/119/2022 | Olanzapine Tablets USP 10 mg (STALANZA 10) | Manufacturer: Stallion Laboratories (Pvt) Ltd, India | 24 000 Packs (10x10's Alu/PVC Blister Pack) | USD 0.75 Per 10x10's Pack) | Permitted as it is a fast moving drug & coming through ICL. Permitted. |
| 26 | 16/08/2022 | SPC/Sri Lanka | Tender No: RES/ICL/01/2022 Indent No: SPC/NDB/168/2022 | Amitriptyline Tablets 10 mg | Manufacturer: Bafna Pharmaceuticals Limnited, India | 15 000 Packs (300 Tablets Per Pack) | USD 1.33 Per Pack | Permitted as dossier is undergoing evaluation & it is coming through ICL. Expedite registration. Permitted. |
| 27 | 18/08/2022 | ABC Pharma Services (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 | Adalimumab Injection (r-DNA origin) 40 mg (AdaliRel) | Manufacturer: Reliance Life Science Private Limited, India | 2 000 Vials | USD 115.00 Per PFS (C&F Sea/Air) | Permitted as it is affordable compared to other products and the dossier has been submitted for registration to NMRA. Expedite registration. Permitted. |
| 28 | 19/08/2022 | SPC/Sri Lanka | 1.) PO No: 90034 Tender No: DHS/P/WW/99/2021 Indent No: DHS/DA/280/2021 2.) PO No: 87720 Tender No: DHS/RP/253/2020 Indent No: DHS/DA/680/2020 | Celecoxib Capsules 100 mg (Cycle 100) | Manufacturer & Supplier: Cadila Healthcare Ltd, India | 25 000 Packs & 70 000 Packs (10x10's Blister Pack) | USD 1.0900 Per Capsule (C&F Price) | Already registered. Not permitted. |
| 29 | 22/08/2022 | Junaht International Pvt. Ltd | PO No: 119268 Tender No: DHS/ICL/X/001/2022 Indent No: DHS/ICL/IG/194/22 Proforma Invoice No: 2022-23/ESO/ESI/00048 | Calcium Gluconate Injection 10% w/v | Manufacturer: Coax Bioremedies Pvt. Ltd, India | 150 000 Ampoules (1's) | USD 0.16 Per Ampoule (C&F Price) | Decision Pending- Documents pending from SPC. |
| 30 | 22/08/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/006/2022 | Cyclosporin Oral Solution USP 100 mg/mL | Manufacturer: Mercury Laboratories Ltd, India | 450 Bottles (50 mL) | USD 81.34 Per Bottle (C&F Air/Sea Price) | Past supplier & through ICL. Permitted. |
| 31 | 22/08/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/007/2022 | Mebeverine Tablets BP 135 mg | Manufacturer: Treffer Pharmaceuticals, India | 120 000 Tablets (1x10's Blister Pack) | USD 1.2073 Per Tablet (C&F Price) | Not peritted as price is unreasonable. Not permitted. |
| 32 | 22/08/2022 | Niix Holdings (Pvt) Limited | Tender No: DHS/P/WW/671/2022 | Tetracosactide Acetate Injection 250 mcg/1 mL (SYNACTHEN) | Manufacturer: Delpharm Tours, UK | 1 000 Ampoules (1 mL) | LKR 26 720.00 Per Ampoule (C&F Price) | Not permitted as there are several discrepancies in the submitted documents. Clarification needed. Not permitted. |
| 33 | 22/08/2022 | Cloud Healthcare (Pvt) Ltd | PO No: 119846 Tender No: DHS/ICL/X/005/2022 Indent No: DHS/ICL/PM/203/2022 | Furosemide Oral Solution USP 20 mg/5 mL (Orsemide) | Manufacturer: Orbit Pharmaceuticals, India | 3 000 Bottles (150 mL) | USD 1.3300 Per Bottle (C&F Price) | Permitted as it is needed at LRH & coming through ICL. Permitted. |
| 34 | 23/08/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/003/2022 | Acyclovir Eye Ointment BP 3% w/w | Manufacturer: Sunways Rohto Pharmaceutical (Pvt) Ltd, India | 2 750 Tubes (5 g) | USD 4.70 Per Tube (C&F Price) | Too high in price. Not permitted |
| 35 | 23/08/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/002/2022 | Daunorubicin Hydrochloride for Injection USP 20 mg (DAUNOMAX) | Manufacturer: Bruck Pharma Private Limited, India | 4 000 Vials | USD 8.13 Per Vial (C&F Sea/Air Price) | Permitted as it is coming through ICL. Permitted. |
| 36 | 23/08/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/002/2022 | Epirubicin Hydrochloride for Injection 10 mg (EPIRUMAX) | Manufacturer: Bruck Pharma Private Limited, India | 250 Vials | USD 21.29 Per Vial (C&F Sea/Air) | Permitted as it is coming through ICL & Oncologists indicated as needed. Permitted. |
| 37 | 23/08/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/006/2022 | Oxytetracycline BP 3% w/w with Hydrocortisone Acetate BP 1% w/w Ear Ointment | Manufacturer: Curis Lifesciences (Pvt) Ltd, India | 10 000 Tubes (15 g) | USD 4.13 Per Tube (C&F Sea/Air) | Permitted as it is coming through ICL. Permitted. |
| 38 | 23/08/2022 | SPC/Sri Lanka | PO No: 112905 Tender No:- DHS/RP/NP/B/361/2021 Indent No: LP/NP/DHS/B/NV/436/2021 | Ledipasvir 90 mg + Sofosbuvir 400 mg Tablets | Manufacturer: Drug International Pharmaceuticals (Pvt) Ltd, Banglasesh | 84 Tablets (1x7's Blister Pack) | LKR 970.0000 Per Tablet (Local) | Permitted as it is below Bangladesh MRP. Permitted. |
| Appeals & Requests of Extensions for the Already Issued WORs, Other | ||||||||
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| A1 | 29/07/2022 | A.Baur & Co. (Pvt) Ltd | Proforma Invoice No: 1259 | Glimepiride Tablets IP 1 mg (AMARYL) | Supplier & Manufacturer: Sanofi India Limited (Goa Site), India | 5 000 Packs of 2x30's Tablets | USD 1.68 | Permitted as it is a site change & renewal is delayed at NMRA. Permitted. |
| A2 | 4/8/2022 | Sunshine Healthcare Lanka Limited | Tender No: RES/07/05/C/2021 Indent No: SPC/EF/064/21 | Pregabalin Capsules 150 mg (Neurobalin 150) | Manufacturer: Sai Mirra Innopharm (Pvt) Ltd, India for Indus Life Sciences (Pvt) Ltd, India | 12 500 Packs of 10x10's PVC Blister Pack | USD 2.17 Per Pack of 10x10's (C&F Price) | Permitted. Extend validity period. |
| A3 | 5/8/2022 | Novachem Lanka (Pvt) Ltd | Tender No: RES/16/06/E/2022 Indent No: SPC/RN/050/2022 | Donepezil Hydrochloride Tablets IP 5 mg (NEPEZ) | Manufacturer: Pharmafabrikon, Unit II, India | 375 Packs of 10x10's | USD 7.10 of 10x10's Pack (C&F Price) | Permitted as it is through ICL. |
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 23/06/2022 | Tabrane Pharmaceuticals (Pvt) Ltd | PO No: 117664 Tender No: DHS/RP/449/2019 Indent No: LP/DHS/MM/3487/2019 | Etoposide Capsules USP 50 mg | Manufacturer: Drug International Ltd, Unit-2, Bangladesh | 1 200 Capsules | LKR 490.00 per Capsule | Paediatric consultant Oncologist confirmed that 50mg is not needed. Can manage with 100mg. Not permitted. |
| 2 | 23/06/2022 | SPC/Sri Lanka | Tender No: DHS/P/WW/669/20 | Methadone Hydrochloride Tablets 5 mg | Supplier: Markss HLC Pvt Ltd, Sri Lanka Manufacturer: Faran Shimi Pharmaceuticals Co-Iran | 32 000 Tablets | LKR 1 475.00/100's | Documents have not been submtted according to the NMRA required channels. No evidence of an established practice & not used as a recommended treatment in Sri Lanka. Not permitted. |
| 3 | 24/06/2022 | Pharma Associates | PO No: 118201 Tender No: DHS/ICL/X/001/2022 Indent No: DHS/ICL/PH/137/2022 | Streptokinase Injection BP 1 500 000 IU | Samarth Life Sciences (Pvt) Ltd, India | 7 000 Vials | USD 12.6000 per Vial of 1 | Discussed with MSD. Since Tenecteplase is very expensive (193,000) & the Cardiologists have agreed to using Streptokinase. A stock has to be maintained at MSD. Samarth Life Sciences (Pvt) Ltd, India is a registered manufacturer at NMRA & it also in the India Tender Procurement list and the price is below Indian tender price of LKR 8,000, this consignment is permitted. No registered supplier of Streptokinase has quoted. Permitted. |
| 4 | 27/06/2022 | Care Ring Pharma (Pvt) Ltd | Tender No: DHS/P/WW/127/22 | Chlorhexidine Gluconate Solution BP/USP 4% W/V | Anphar Organis (Pvt) Ltd, India | 12 000 Bottles | USD 2.20 per 500 mL Bottle | Applicant not registered. Not permitted. |
| 5 | 28/06/2022 | SPC/Sri Lanka | Tender No: RES/16/06/E/2021 Indent No: SPC/RN/050/2022 | Donepezil Hydrochloride Tablets IP 5 mg | Pharmafabricon, India | 375 Packs | USD 7.10 Per 10x10's Pack | All documents not attached. Registration has expired. Several quality failures. Not permitted. |
| 6 | 28/06/2022 | SPC/Sri Lanka | Tender No: RES/28/07/A/2021 Indent No: SPC/CR/062/2022 | Chlorpromazine Tablets BP 50 mg | BDH Industries Limited, India | 2 500 Packs | USD 14.40 Per Pack | BDH has several quality failures. Not permitted. |
| 7 | 1/7/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/002/2022 | Thalidomide Capsules USP 100 mg | Bruck Pharma Pvt. Ltd, India | 100 000 Capsules | USD 1.2175 Per Capsule | All documents not supplied. Should supply all documents before letter is issued. Permitted. |
| 8 | 4/7/2022 | SPC/Sri Lanka | Tender No: DHS/RP/449/2019 Indent No: LP/DHS/MM/3487/2019 PO No: 117664 | Etoposide Capsules USP 50 mg (TOPOSAR) | Drug International Ltd, Bangladesh | 1 200 Capsules | LKR 490.00 per Capsule | Not required. Not permitted. |
| 9 | 8/7/2022 | SSK Pharma (Pvt) Ltd | Tender No: DHS/RP/46/2022 | Fluconazole Injection USP 200 mg/ 100 mL (FLUCOHEM) | Captab Biotech, India | 47 000 Vials | LKR 189..00 per 100 mL | Applicant & manufacturer not registered. Not permitted |
| 10 | 8/7/2022 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: SHL/02-REG/22 | Dapagliflozin Propanediol Monohydrate Tablets 5 mg (DFLOZIN 5) | Hilton Pharma (Pvt) Ltd, Pakistan | 24 000 Packs | USD 1.260 (Unit Price) | Expedite registration. Not permitted. |
| 11 | 8/7/2022 | Sunshine Healthcare Lanka Ltd | Proforma Invoice No: SHL/02-REG/22 | Dapagliflozin Propanediol Monohydrate Tablets 10 mg (DFLOZIN 10) | Hilton Pharma (Pvt) Ltd, Pakistan | 24 000 Packs | USD 1.960 (Unit Price) | Expedite registration as only 3 manufacturers are registered. Not permitted. |
| 12 | 8/7/2022 | Tabrane Pharmaceuticals (Pvt) Ltd | Tender No: DHS/RP/222/2021 Indent No: LP/DHS/PH/3485/2021 PO No: 116104 | Etoposide Capsules USP 100 mg (TOPOSAR 100) | Drug International Ltd, Bangladesh | 8 750 Capsules | LKR 770.00 Per Capsule | No registered manufacturer. Permitted for this consignment. Should register product for future. Permitted. |
| 13 | 8/7/2022 | Pharma Associates | Tender No: DHA/ICL/X/005/2022 | Venlafaxine Hydrochloride Extended Release Capsules 75 mg | Bafna Pharmaceuticals Ltd, India | 6 200 000 Capsules | USD 5.50 Per 100 Capsules | Bafna has had quality failures in the past. This product has come from a different site which has a GMP approval from MHRA, UK. Coming thorugh Indian Credit Line. Permitted. |
| 14 | 11/7/2022 | Novachem Lanka (Pvt) Ltd | Tender No: DHS/ICL/X004/2022- Proforma Invoice No: PROFORMA/09 Dated 28/06/2022 | Ketorolac Tromethamine Sterile Ophthalmic Solution 0.4%z | Modern Laboratories, India | 35 000 Vials | USD 1.80 Per 1's | Not a registered suppliers, but permitted as it is from an Indian credit line. Applicant is registered. Permitted. |
| 15 | 12/7/2022 | SPC/Sri Lanka | Tender No: RES/ICL/12/2022 | Erythromycin Stearate Tablets BP 500 mg | Belco Pharma, India | 4000 Packs | USD 6.3 Per Pack (C&F Price) | Not permitted as manufacturer has had several quality failures. Not Permitted |
| 16 | 12/7/2022 | Ceyoka (Pvt) Ltd | Tender No: DHS/ICL/X/005/2022 | Ketamine Hydrochloride Injection USP 10 mg/mL | Ciron Drugs & Pharmaceuticals (Pvt) Ltd, India | 12 000 Vials | USD 3.15/1's (C&F Price) | Permitted as other strengths are registered. Permitted. |
| 17 | 12/7/2022 | Ceyoka (Pvt) Ltd | Tender No: DHS/ICL/X/004/2022 | Brimonidine Eye Drops 0.15% | Ciron Drugs & Pharmaceuticals (Pvt) Ltd, India | 43 000 Vials | USD 0.89/Vial (C&F Price) | Not permitted as the strengths are different. Not permitted. |
| 18 | 12/7/2022 | Hemas Pharmaceuticals (Pvt) Ltd | Sri Lanka/Hemas/Tacro/PFI1 | Tacrolimus Capsules 1 mg (MYLIMUS 1) | Concord Biotech Ltd, India for Mylan Pharmaceuticals (Pvt) Ltd, India | 30 000 Packs | USD 1.25 Per Pack (CIF Price) | Send through donation channel. Not permitted. |
| 19 | 12/7/2022 | Hemas Pharmaceuticals (Pvt) Ltd | Sri Lanka/Hemas/Tacro/PFI1 | Tacrolimus Capsules 0.5 mg (MYLIMUS 0.5) | Concord Biotech Ltd, India for Mylan Pharmaceuticals (Pvt) Ltd, India | 16 000 Packs | USD 0.90 Per Pack (CIF Price) | Send through donation channel. Not permitted. |
| 20 | 12/7/2022 | Markss HLC (Pvt) Ltd | PO No: 114870 Tender No: DHS/RP/NP/O/172/2021 Indent No: LP/NP/DHS/O/PM/480/21 | Nivolumab Injection 100 mg/10mL (OPDIVO) | Bristol Mayer Squibb, USA | 24 Vials | LKR 160 000.0000 Per Vial of 1 | Send to high priced committee. Not permitted. |
| 21 | 15/07/2022 | Yaden International (Pvt) Ltd | Tender No: RES/ICL/12/2022 | Erythromycin Ethyl Succinate Oral Suspension BP 125 mg/5 mL | Centurion Remedies (Pvt) Ltd, India | 12 000 Bottles | USD 0.85 Per 100 mL Bottle | Not permitted. Get from registered manufacturer. |
| 22 | 19/07/2022 | Cloud Healthcare (Pvt) Ltd | Tender No: DHS/ICL/X/005/2022 Indent No: DHS/ICL/MM/152/2022 PO No: 118804 | Lidocaine and Prilocaine Cream BP 5% w/w (Orprilo) | Orbit Pharmaceuticals, India | 7 000 Tubes | USD 0.6000 Per Tube of 1 (Unit Price (C&F)) | Both applicant & manufacturer are not registered. Not permitted. |
| 23 | 22/07/2022 | Yaden International (Pvt) Ltd | Tender No: DHS/ICL/X/006/2022 | Sumatriptan Tablets BP 50 mg (SUMITOP-50) | Centurion Laboratories (Pvt) Ltd, India | 7 000 Tablets | USD 15.5685 Per Tablet (Unit Price (C&F)) | The price quoted is very high. MRP in Sri Lanka is approximately LKR 50/= - 60/= Not permitted. |
| Appeals & requests of extensions to the already issued WORs, Other | ||||||||
| Serial Number | Date of Submission | Applicant | Qutation number/ PO No. Tender No./Indent No. /Invoice | Product | Supplier/Manufacturer | Quantity | Unit Price | Decision of WOR Committee |
| 1 | 24/06/2022 | SPC/Sri Lanka | Indent No: DHS/M/PM/069/2022 | Quadrivalent Human Papilloma Virus (Types 6,11,16,18) Recombinanat Vaccine Single Dose Vial (GARDSIL) | Supplier: United Nations Children's Fund, Denmark Manufacturer: Merck Sharp & Dohme Corpe., USA/Netherland | 400 000 Vials | Extend original. Permitted. | |
| 2 | 30/06/2022 | Talex Pharma (Pvt) Ltd | Indent No: LP/DHS/AMS/3420/22 Tender No: DHS/WW/152/22 | Melatonin Tablets 2 mg | Supplier: Talex Pharma (Pvt) Ltd Manufacturer: Davis Pharmaceuticals Laboratories, Pakistan | 170 000 Tablets | LKR 13.2000 Per- Tablet of 1 | Extend Permition. Permitted. |
| 3 | 5/7/2022 | SPC/Sri Lanka | Tender No: DHS/P/WW/232/20 Indent No: LP/DHS/IN/2936/20 | Diazoxide Tablets 50 mg | Supplier: Yaden International- Pvt Ltd, Sri Lanka Exporter: Prime Pharmacare Ltd, U.K. Manufacturer: RPH Pharmaceuticals AB, Sweden | 18 000 Tablets | LKR 238.0000 Per Tablet of 1 | Extend Permit. Permitted. |
| 5 | 11/7/2022 | George Steuart Health (Pvt) Ltd | Proforma Invoice No: EXP094/22 | Dextran 40 in Sodium Chloride Injection | Manufacturer: Thai Otsuka Pharmaceutical Co. Ltd | 200 Bags | USD 24.50 (C&F Price) | Permitted for this consignment only as there is no dextran in the private sector. Permitted. |
| 6 | 11/7/2022 | George Steuart Health (Pvt) Ltd | Proforma Invoice No: SAMPLE/EFP/002 | Rabies Antiserum (Premi-RIG) | Manufacturer: Premium Serums and Vaccines Private Limited, India | 40 Vials | USD 1 Per Vial (Unit Rate) | Send to donation pathway. Not permitted. |
| 7 | 20/06/2022 | SPC/Sri Lanka | Tender No: DHS/RP/338/2020 Indent No: DHS/IN/690/20 Commercial Invoice No: EXS0017 | Gabapentin Capsules USP 100 mg (RAVASTAL 100) | Manufacturer: Stallion Laboratories (Pvt) Ltd, India Local Agent: Siba Healthcare (Pvt) Ltd | 2 000 000 Capsules | USD 0.9700 Per Capsule (Unit Price (C&F)) | MSD has issued a letter that shelflife is acceptable. Permitted. |
Summary of the decisions taken at the Subcommittee for Waiver of Registration - Medicines approved by the 67th Medicine Evaluation Committee
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No./Invoice No. | Product | Supplier/Manufacture | Qty | Unit Price | Decision |
| 1 | 6-Jun-22 | SPC/Yaden International | DHS/RP/472/2020 | Phytomenadione Tablets BP 5mg | Mercury Laboratories Ltd, India (Supplier: Eureka Life Sciences Pte. Ltd, Singapore) | 49,000 Tablets (100's) | 0.49 USD/Tablet (49.00 USD/100 Tablets) | The MSD confirmed that it is needed SPC has postponed the decision - Not permitted |
| 2 | 6-Jun-22 | Tabrane Pharmaceuticals Pvt. Ltd | LP/NP/DHS/B/NV/436/2021 | Ledipasvir 90mg + Sofosbuvir 400mg Tablets (HOPSO-LP) | Drug International Pharmaceuticals (Pvt) Ltd, Bangladesh | 84 Tablets (1x7's) | 970.00 LKR/Tablet | This is for a single patient according to the information provided by MSD. Hence the committee needs a justification from the consultant.- Decision pending |
| 3 | 6-Jun-22 | Hemas Pharmaceuticals | SPC/PG/087/2021 (1055000349) | Warfarin Sodium Tablets 1mg (ZOFARIN 1) | Cadila Healthcare Ltd, India | 8,135 Packs (100's) | 0.015 USD / Tablet (1.50 USD/100's) | Expedite the registration of product due to site change.- Not permitted |
| 4 | 8-Jun-22 | Emerchemie NB (Ceylon) Ltd | LP/NP/DHS/O/SP/383/2021 | Pembrolizumab Injection 25mg/ml (Pembroxim) | Beacon Pharmaceuticals, Bangladesh | 34 vials (4ml) | 415,000.00 LKR/Vial | This is a high priced Medicine. College of Oncologists has provided a needed list. Pembrolizumab is not in the needed list. There is no letter of justification by the consultant - Not permitted |
| 5 | 8-Jun-22 | Markss HLC (Pvt) Ltd | LP/NP/DHS/O/PM/510/21 | Cladribine Injection 1mg/ml vial (CLADRIM) | Fresenius Kabi India Ltd, India | 26 vials (10ml) | 42,000 LKR/Vial | This is a high priced medicine not included in the products needed by the College of Oncologists. Hence not permitted as there is no justification from consultant- Not permitted |
| 6 | 8-Jun-22 | Markss HLC (Pvt) Ltd | DHS/P/WW/669/20 | Methadone Hydrochloride Tablets 5mg | Faran Shimi Tuyserkan Co., Iran | 16,000 tablets (100's) | (1,475.00 LKR /100's) (14.75LKR/Tablet) | This is for a GP in the private sector. The manufacturer and the product is not registered. If there is a need, Methadone should be registerd. Priority registration will be recommended.- Not permitted |
| 7 | 8-Jun-22 | Markss HLC (Pvt) Ltd | LP/NP/DHS/B/PM/396/21 | Cladribine Injection 1mg/ml (CLADRIM) | Fresenius Kabi India Ltd, India | 24 vials (10ml) | 38,000 LKR/Vial | High priced medicne. Not included in the "Needed" list provided by College of Oncologists. No justification provided by consultant.- Not permitted |
| 8 | 24-May-22 | A. Baur & Co. (Pvt) Ltd | MPPL/Sri Lanka / Mylan/P/2022/01 | Enoxaparin Sodium Injection IP 40mg/0.4ml (KAIVEXIN-40) | Soveregin Pharma Pvt. Ltd, India | 50,000 Vials (0.4ml) | - | Not a registerd product. No justification provided regarding need of the product. Several registerd products available.- Not permitted |
| 9 | 1-Jun-22 | A. Baur & Co. (Pvt) Ltd | 1200011993 | Capacitabine Tablets 500mg (Xeloda) | Excella GmbH, Germany for F. Hoffmann-La Roche Ltd, Switzerland | 100 Packs (120's) | - | Expedite registration if submitted as it is a EU GMP registerd manufacturing site.- Not permitted |
| 10 | 6-Jun-22 | Omacx Healthcare (Pvt) Ltd | Proforma/25052022 | Sterile Water for Injection 5ml | Sunlife Pharma Services, India | 102,000 Packs (5ml) | - | Not permitted as it is not a registerd product- Not permitted |
| 11 | 6-Jun-22 | A. Baur & Co. (Pvt) Ltd | 1259 | Glimepiride Tablets IP 1mg (AMARYL) | Sanofi India Ltd (Goa Site), India | 5,000 Packs(2x30's) | - | Expedite site change approval- Not permitted |
| 12 | 8-Jun-22 | Majstro Health (Pvt) Ltd | RP/PI/22-23/01 | Atracurium Besylate Injection USP 10mg/ml | Kwality Pharmaceuticals Ltd, India | 500,000 Ampoules (2.5ml) | - | Not permitted as both the applicant & product are not registerd- Not permitted |
| 13 | 10-Jun-22 | Emar Pharma (Pvt) Ltd | WPPL/30/2021-22 | Ivermectin Tablets 12mg (IVERACE) | Mascot Health Series Pvt. Ltd, India for Wallace Pharmaceuticals (Pvt) Ltd, India | 2,500 Packs (10x10's) | - | Permitted for this consignment as it has already been approved- Permitted |
| 14 | 10-Jun-22 | Yaden International (Pvt) Ltd | LP/DHS/WD/2632/20 | Diazoxide Tablets 50mg | RPH Pharmacueitcal AB, Sweden | 9,000 tablets | - | Request price for consideration- Decision pending |
| 15 | 10-Jun-22 | Sunshine Healthcare Lanka Ltd | DHS/WD/140/21 | Linezolid Tablets 600mg (LIZOLID-600) | Glenmark Pharmaceuticals Ltd, India | 30,000 tablets (20's) | 0.3845 USD/Tablet (7.69USD/20's) | Permitted for this consignment as it is a registered product & there is a delay in submission of dossiers for registration renewal due to site change- Permitted |
| 16 | 10-Jun-22 | SPC | DHS/AR/432/2021 | Meningococcal Vaccine Single Dose Vial (Nimenrix) | Pfizer Manufacturing Belgium NV, Belgium (Supplier: Pfizer Export B.V. - Netherlands) | 5,000 Vials (1's) | 27.70 USD/Vial | Permitted as there is no registerd product only for this consignment - Permitted |
| 17 | 10-Jun-22 | SPC/Niix Holdings Pvt. Ltd | LP/DHS/PM/3271/21 | Cocaine Hydrochloride Solution 10% | Macarthy Laboratories Ltd, UK | 1,000 bottles (2.5ml) | - | Price has not been stated- Decision pending |
| 18 | 15-Jun-22 | SPC/Hemas Pharmaceuticals | DHS/MM/475/2021 | Fenofibrate Capsules USP 200mg (LIPICARD) | USV Pvt. Ltd, India | 499,996 Capsules (4x7's) | - | Expedite registration- Not permitted |
| 19 | 20-Jun-22 | Markss HLC (Pvt) Ltd | PLL-X-2223-12 | Prednisolone Syrup 5mg/5ml (PREDKID) | Paranax Lab Ltd, India | 40,000 Bottles (60ml) | - | Expedite registration- Not permitted |
| 20 | 20-Jun-22 | SPC/Siba Healthcare | DHS/IN/690/20 | Gabapentin Capsules USP 100mg (RAVASTAL-100) | Stallion Laboratories (Pvt) Ltd, India | 2,000,000 capsules (10x10's) | - | To get a letter from MSD stating that the short shelf life is acceptable- Decision pending. MSD has issued a letter that shelflife is acceptable. Permitted. |
| 21 (i) | 22-Jun-22 | George Steuart Health (Pvt) Ltd | SAMPLE/EFP/002 | Combipack of Snake Venom Antiserum IP with Sterile water for Injection IP | Premium serums and vaccines (Pvt) Ltd - India | 40 vials (10ml) | Expedite evaluation of submitted registration dossier. SPC & MSD informed that sufficient stocks are available in the state sector. Not permitted | |
| 21 (ii) | 22-Jun-22 | George Steuart Health (Pvt) Ltd | SAMPLE/EFP/002 | RABIES Antiserum (Premi-RIG) 1000 IU/5ml | Premium serums and vaccines (Pvt) Ltd - India | 40 vials (5ml) | Rabies Antiserum dossier has not been submitted. Not permitted | |
| 22 | 22-Jun-22 | George Steuart Health (Pvt) Ltd | EXP 094/22 | Detran 40 and Sodium Chloride injection (Dextran 40 Injection) | Thai Otsuka Pharmaceutical Co. LTD - Thailand | 200 bags (500ml) | - | Not permitted as it is locally manufactured. No justification has been provided for importing the product- Not permitted |
| 23 | 22-Jun-22 | Sunshine Healthcare Lanka Ltd | 64 | Pantoprazole for IV injection 40mg (PANTODAC IV) | Cadila Healthcare Ltd, India | 10,000 Vials (1's with diluent) | Expedite sample license process & ask for dossier for evaluation. Registered products are available- Not permitted | |
| 24 | 22-Jun-22 | Yaden International (Pvt) Ltd | DHS/ICL/X/003/2022 | Flucloxacillin Oral Solution BP 125mg/5ml | Sparsh Bio-tech Pvt Ltd- India | 150,000 bottles (100ml) | 1.5568 USD/Bottle | MSD to justify why this is needed- Decision pending |
| 25 | 15-Jun-22 | ABC Pharma services (Pvt) Ltd | 33/RLS/ABC/05/2022-23 | Tenecteplace (TNK-tPA) Lyophilized powder for Solution for Injection 40mg (Tenecterel 40mg) | Reliance Life Science (Pvt) Ltd- India | 2,000 Vials (1's) | - | To arrange a meeting with applicant as soon as possible to discuss- Decision pending |
| 26 | 22-Jun-22 | Tabrane Pharmaceuticals (Pvt) Ltd | DHS/RP/242/2021 | Megestrol Acetate TabletsUSP 40mg (Apit-40) | Drug International Ltd Unit-2 - Bangladesh | 5,000 Tablets (10x10's) | 970.00LKR/Tablet | Tabrane has a registered product. To purchase from registered manufacturer. This product is not registered at NMRA- Not permitted |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No./Invoice No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 11-Mar-22 | Lionchem Pvt. Ltd | LP/DHS/L/WW/115/260CPW/2020 | Sodium Chloride | Sisco Research Laboratories (Pvt) Ltd, India | 600 Pcs (500g) | 1.08 USD | This is not within the TOR of WOR committee. Not permitted. |
| 2 | 11-Mar-22 | Sunshine Healthcare Lanka Ltd | DHS/P/WW/551/2020 | Adapalene Gel 0.1% | Glenmark Pharmaceutical Ltd, India | 24,000 tubes | 0.29 USD | WOR cannot be given as MRP has been negotiated with the MAH. Not permitted. |
| 3 | 11-Mar-22 | SPC/Ravilux Company | DHS/DA/455/2021 | Dexamethasone Tablets 4mg | BDH Industries Ltd, India | 180,000 tablets | - | Extension of previous WOR. Permitted. |
| 4 | 11-Mar-22 | SPC/Ravilux Company | DHS/DA/451/2021 | Dexamethasone Tablets 8mg | BDH Industries Ltd, India | 90,000 tablets | - | Extension of previous WOR. Permitted. |
| 5 | 11-Mar-22 | Sunshine Healthcare Lanka Ltd | DHS/P/WW/652/19 | Linezolid IV Injection 200mg/100ml | Glenmark Pharmaceutical Ltd, India | 5,500 Bags | 2.48 USD/Bag | Expedite registration. Not permitted. |
| 6 | 11-Mar-22 | SPC/ABC Pharma Services Pvt. Ltd | SPC/PT/065/2021 | Fluorometholone Ophthalmic Suspension USP 0.1% | Popular Pharmaceuticals Ltd, Bangladesh | 3,000 Bottles / 5ml | 0.60 USD/5ml Bottle | Expedite registration. Not permitted. |
| 7 | 11-Mar-22 | SPC/Markss HLC Pvt. Ltd | DHS/P/WW/307/2022 | Ritonavir Tablet 100mg | Mylan Laboraotires Ltd, India | 50,520 tablets | 2,500 LKR/30's | Expedite registration. Not permitted. |
| 8 | 11-Mar-22 | SPC/Pharmatec Pvt. Ltd | DHS/MM/159/2021 | Dexamethasone Injection USP 8mg/2ml | Belco Pharma, India | 500,000 Ampoules | 2.70 USD/Ampoule of 10 | Expedite registration. Not permitted. |
| 9 | 11-Mar-22 | SPC/Pharmatec Pvt. Ltd | DHS/MM/223/2021 | Neostigmine Injection 0.5mg/ml | Belco Pharma, India | 55,000 Ampoules | 2.70 USD/Ampoule of 10 | Permitted as higher strength is registered & there is a crisis due to shortage. Permitted. |
| 10 | 11-Mar-22 | SPC/A Baur & Co. Ltd | DHS/NV/596/2020 | Moxifloxacin Hydrochloride Eye Drops 0.5% | Ajanta Pharma Ltd, India | 20,000 Bottles | 0.49 USD/5ml Bottle | Expedite registration. Not permitted. |
| 11 | 11-Mar-22 | AVH Distributors Pvt. Ltd | DHS/RP/NP/B/447/2021 | Vandetanib 100mg Tablets | Tizig Pharma Ltd, Nepal | 540 tablets | 3,880 LKR/Tablet | Manufacturer not registered at NMRA. Should apply for registration. Not permitted. |
| 12 | 11-Mar-22 | Yaden Intrnational | DHS/RP/174/2021 | Calcium with Vitamin D3 Tablets 500mg 250 IU | Bal Pharma Ltd, India (Supplier : Eureka Life Sciences Pvt. Ltd, Singapore) | 6,000,000 tablets | 0.76 USD/100's | Not an emergency Medicine. Registered products avialable. Not permitted. |
| 13 | 15-Mar-22 | The Schazoo Pharmaceutical Laboratories Pvt. Ltd, Pakistan/Edna Medicals Pvt. Ltd | DHS/P/WW/700/21 | Sodium Chloride 5% Eye drops | The Schazoo Pharmaceutical Laboratories Pvt. Ltd, Pakistan | 18,750 Bottles | 3.47 USD/Pack | Registered & local products available. Not Permitted. |
| 14 | 15-Mar-22 | CCECC-CRCG Joint Venture Pvt. Ltd | - | Arbidol Hydrochloride Tablets, Rabavirin capsules, Azithromycin Tablets, Cefixime Capsules,Mixture Qing Fei Pai Du, Tablets Inflammation of the lungs, D-Fense 2 (concentrated herabl extract tea), Huashi Baidu kala | - | As per the inovice | - | Not within our TOR. Not permitted. |
| 15 | 24-Mar-22 | Trojan Ceylon Group Pvt. Ltd | DHS/RP/432/2020 | Lidocaine Topical Aerosol USP 10% | Dortmund Pharmaceuticals, India | 4,000 bottles | 725.00 LKR/Bottle (50ml) | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 16 | 22-Mar-22 | Markss HCL Pvt. Ltd | DHS/P/WW/300/22 | Lopinavir 100mg + Ritonavir 25mg Tablets | Mylan Laboraotires Ltd, India | 48,600 tablets | 5,350 LKR/60's | STD indicated that it is not needed. Not permitted. |
| 17 | 22-Mar-22 | Yaden Intrnational | LP/DHS/WD/3340/21 | Benztropine Mesylatre Injection USP 2mg/2ml | Kwality Pharmaceuticals Ltd, India | 2,000 Ampoules | 10,930.00 LKR / Ampoule | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 18 | 22-Mar-22 | Yaden Intrnational | DHS/RP/473/2020 | Folinic Acid Tablets USP 15mg (Leucovorin Calcium Tablets USP 15mg) | Neon Laboratories Ltd, India | 22,000 tablets | 31.00 USD/30's | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 19 | 24-Mar-22 | Tabrane Healthcare Pvt Ltd | (i) LP/NP/DHS/B/NV/389/2021 (ii) LP/DHS/B/NV/393 | Ribavirin Capsules USP 200mg | Julphar Bangladesh Ltd, Bangladesh | (i) 60 Capsules (ii) 60 Capaules | 990.00 LKR & 970.00 LKR | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 20 | 24-Mar-22 | Chensa Pharmaceuticals Pvt. Ltd | DHS/RP/NP/O/437/2021 | Olaparib Capsules 50mg | Everest Pharmaceuticals Ltd, Bangladesh | 39,984 Capsules | 285.00 LKR/Capsule | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 21 | 24-Mar-22 | Chensa Pharmaceuticals Pvt. Ltd | DHS/RP/NP/O/305/21 | Olaparib Capsules 50mg | Everest Pharmaceuticals Ltd, Bangladesh | 48,496 Capsules | 247.00 LKR/Capsule | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 22 | 24-Mar-22 | SPC/Yaden International | LP/DHS/WD/2632/20 | Dobutamine Injection USP 250mg / 20ml | Mercuy Laboratories Ltd, India | 70,000 vials | 283.00 LKR/Vial | Expedite registration. Not permitted. |
| 23 | 24-Mar-22 | SPC/Emerchemie NB (Ceylon) Ltd | (i) LP/NP/DHS/B/E/345/2021 (ii) LP/NP/DHS/B/E/346/2021 | Mycophenolic Sodium Tablet 360mg | Emcure Pharmaceuticals Ltd, India | (i) 1,080 Tablets (ii) 180 Tablets | 2249.58 LKR/ 10's | Expedite registration. Not permitted. |
| 24 | 24-Mar-22 | SPC/Emerchemie NB (Ceylon) Ltd | DHS/RP/175/2021 | Propylthiouracil Tablets BP 50mg | Macleods Pharmaceuticals Ltd, India | 475,000 Tablets | 7.88 USD/100's | MSD not participated to this meeting. Hence, sheduled for next (May-2022) commiittee meeting. |
| 25 | 11-Mar-22 | SMM Halcyon Pvt. Ltd. | DHS/RP/NP/B/384/2021 | Tenofovir Alfenamide Tablets 25mg | Mylan Laboraotires Ltd, India | 90 Tablets | 200.00 LKR/ Tablet | As no registered product & price is ok. Permitted. |
| 26 | 6-Apr-22 | Tabrane Pharmaceuticals (Pvt) Ltd | 039/TABRANE/05-2022 (TABRANE-15/2021) | Remdesivir Lyophilized Powder for Ivh | Drug Intenrational Ltd, Bangladesh | 10,000 Vials (1,000 vials) | 8.50 USD / Vial | Not permitted. Not approved by covid evaluation committee. |
| 27 | 6-Apr-22 | Markss HCL Pvt. Ltd | DHS/P/WW/306/22 | Darunavir (as Thanolate) 600mg Tablet | Mylan Laboraotires Ltd, India | 19,440 Tablets | 25,050.00 LKR / 60's | As not registered product & price is acceptable. Permitted. |
| 28 | 6-Apr-22 | SPC/Slim Pharmaceuticals | LP/DHS/AR/3388/2022 | Recombinant Human Erythropoietin Injection 10,000 IU/ml | Shandong Kexing Bioproducts Co. Ltd, China | 25,299 PFSY | 1170.00 LKR/ 1 PFSY | Still under pricing negoatitation. Not permitted. |
| 29 | 6-Apr-22 | SPC/Sanofi Lanka Ltd | DHS/AR/675/2019 | Meningococcal (Group A.C.Y and W-135) Polysacchride Diphtheria Toxoid Conjegate Vaccine 0.5ml vial | Sanofi Pasteur Inc - USA (Suplier : Sanofi Healthcare India Pvt. Lrd, India) | 750 vials | 33.00 USD /Vial | No registered products. Permitted. |
| 30 | 4-Apr-22 | National STD/AIDS Control Programme | PO No. 202811657 | Nevirapine Suspension 5mg / 5ml - 100ml | Aurobindo Pharma Ltd, India | 250 bottles | - | Free of Charge |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No./Invoice No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 04-Mar-22 | Sunshine Healthcare | L2236 | Olmesartan Medomoxil 20mg Tablets | Macleods Pharmaceuticlas Ltd, India | 3,620 Packs (4x7's) | - | Approved only for this consignment as it is a registered product at the NMRA & the shipment has arrived (Permitted) |
| 2 | 04-Mar-22 | Sunshine Healthcare | NSHXO221S00071 | Adapalene 1% + Benzyl Peroxide 2.5 Gel | Encube Ethical Pvt. Ltd, France | 6,000 Tubes (30g) | - | Permitted only for this shipment. NMRA to expedite the process. (Permitted) |
| 3 | 04-Mar-22 | Pharma Associates | DHS/P/WW/18/2022 | Methylene Blue Injection 1% w/v 10ml Ampoules | VEM ILAC S., Turkey | 4,000 Ampoules | 6,500 LKR / Ampoule | To have a meeting with MAH. (Not Permitted) |
| 4 | 18-Feb-22 | Microtech Biological Pvt. Ltd | Quotation No. ASP/22011825$ | Mouse Monoclonal [HBME-1] to Mesothelioma-Abcam | Abcam, United Kingdom (Dis. by Allied Scientific Products, India) | 1 (500ul) | 219,500 LKR/Unit price | Research protocol should be submitted. (Decision Pending) |
| 5 | 18-Feb-22 | Yaden International Pvt. Ltd | LP/DHS/WD/2632/20 | Dobutamine Injection USP 250mg / 20ml | Mercury Laboratory Ltd, India | 35,000 Vials | 283.00 LKR/Vial | Batch failure has been reported from the same manufacturer in September 2021. Recall tender. (Not Permitted) |
| 6 | 18-Feb-22 | Pharmatec Pvt. Ltd | DHS/RP/04/21 | Neostigmine Injection 0.5mg/ml | Belco Pharma Ltd, India | 25,000 Ampoules (10ml x 1) | 2.70 USD/Ampoules of 10 | Several registerd products available. (Not Permitted) |
| 7 | 18-Feb-22 | SMM Halcyon (Pvt) Ltd | LP/P/CPU/DHS/TEP/024/2020 | Valpatasvir 100mg & Sofosbuvir 400mg Tablets | Mylan Laboratories, India | 84 Tablets (28x1) | 30,000 LKR/28's | Permitted as they have applied for registration. (Permitted) |
| 8 | 23-Feb-22 | A Baur & Co. Ltd | 1200011754 | Capecitabine Tablets 500mg | Excella GmbH, Germany for F. Hoffmann-La Roche Ltd, Switzerland | 150 Packs | - | Oncologist confermed that it is needed. (Permitted) |
| 9 | 23-Feb-22 | Orexo Biopharma (Pvt) Ltd | LP/DHS/HD/2822/2020 | Cholecalciferol Tablets 5000IU | Solitaire Pharmacia Pvt. Ltd, India | 1,000 Packs (10x10) | 40.00 LKR/Tablet | Not needed urgrntly & sufficient registerd products available. (Not Permitted) |
| 10 | 23-Feb-22 | Orexo Biopharma (Pvt) Ltd | LP/DHS/PS/2823/2020 | Cholecalciferol Tablets 1000IU | Solitaire Pharmacia Pvt. Ltd, India | 200,000 Capsules | 22.00 LKR/Capsule | See remark in (9) SPC indicated] it is not needed. (Not Permitted) |
| 11 | 23-Feb-22 | SPC | DHS/M/PM/069/2022 | Quadrivalent Human Papi;;omavirus (HPV) Vaccine 4-Valent Single Dose vial with Vaccine vial monitor 9VVM) | Merck Sharp & Dohme Corp., USA/Netherland (Supplier: United Nations Children's Fund - Denmark) | 400,000 Vials | 4.50 USD/Vial | Permitted |
| 12 | 23-Feb-22 | ABC Pharma | DHS/P/WW/399/18 | Human Varicella-zoster immunoglobulin 25IU/ml, 5ml solution for Infusion | Biotest Pharma GmbH, Germany | 90 Ampoules | - | Permitted as it is needed in the country. (Permitted) |
| 13 | 23-Feb-22 | Hemas Pharmaceuticals | DHS/IG/627/18 | Cyclosporine Sterile Ophthalmic Emulsion 0.05% w/v | Aristo Pharma Ltd, Bangladesh | 1500 Bottles | 2.98 USD/Bottle | Not needed by SPC. (Not Permitted) |
| 14 | 23-Feb-22 | Sunshine Healthcare | SCP/EF/064/21 | Pregabalin Capsules 150mg | Sai Mirra Innopharm Pvt. Ltd, India for Indus Life Sciences Pvt. Ltd, India | 12,500 Packs (10x10's) | 2.17 USD/10x10's | Extend registration. Permitted for this consignment. (Permitted) |
| 15 | 23-Feb-22 | Yaden International Pvt. Ltd | DHS/RP/442/2019 | Zuclopenthixol Decanoate Injection BP 200mg/ml | Mercury Laboratory Ltd, India (Supplier: Eureka Life Sciences v Pte. Ltd, Singapore) | 300 Ampoules | 25.60 USD/Ampoule | Check registration status. Cost is high. (Not Permitted) |
| 16 | 23-Feb-22 | A Baur & Co. Ltd | 1244 | Glimepiride Tablets IP 3mg | Sanofi India Ltd, India | 1,500 Packs (2x30) | Approved as new site is registerd. (Permitted) | |
| 17 | 23-Feb-22 | Care Ring Pharma Pvt. Ltd | LP/P/CPU/DHS/O/E/002/2019 | Panitumumab Injection 100mg/5ml | Amgen Inc. - USA | 144 Vials | 155,500.00 LKR/Vial | Send to high cost to check cost - effectivenese . Oncologist Recommendation. (Not Permitted) |
| 18 | 23-Feb-22 | A Baur & Co. Ltd | 30001234 | Theophylline 26.67 mg per 5ml Syrup | Ensign Laboratories Pty. Ltd, Australia for iNova Pharmaceuticals, Singapore | 29,040 Packs + 3,300 Packs (100ml / 500ml) | Permitted for this consignment as delay is due to NMRA. Please expedite renewal of registration. (Permitted) | |
| 19 | 23-Feb-22 | Sunshine Healthcare | SPC/NDB/082/2021 | Amlodipine Besylate Tablets USP 10mg | Cadila Healthcare Ltd, India | 12,000 Packs (10x10's) | Not Needed. (Not Approved) | |
| 20 | 23-Feb-22 | A Baur & Co. Ltd | 13190LK | Ibandronic Acid Tablets 150mg | Penn Pharmaceutical Services Ltd, UK | 300 Packs | - | Site change. Site registered with NMRA. (Permitted) |
| 21 | 23-Feb-22 | Yaden International Pvt. Ltd | DHS/RP/180/2021 | Hydoxyrogesterone Carroate Injection USP 250mg/ml | Zafa Pharmaceuticals Laboratories (Pvt) Ltd, Pakistan (Supplier: Eurekas Lifesciences, Singapore) | 15,000 Ampoules | 1.93 USD/Ampoule | Manufacturer has not supplied critical data for registration. (Not Permitted) |
| 22 | 23-Feb-22 | SPC/Supplier : Belco Pharma, India | DHS/HD/390/2019 | Administration Device set + Sodium Chloride 0.9%, w/v 500ml Glass Bottle for every six vials of Paclitaxel Injection USP 6mg in 5ml vial | For Diluent - Albert David Ltd-India, For Administration Device - Angiplast Pvt. Ltd, India | 4,500 - Administration Device Sets + 4,500 Sodium Chloride 0.9% w/v 500ml Glass Bottle | 16.2 USD/Vial | Permitted as the product is registered and there is a shorting at MSD. (Permitted) |
| 23 | 04-Mar-22 | A Baur & Co. Ltd | QU-0120 | Vitamin C 500g and Vitamin D3 1000mg | AussieSupps Pty Ltd, Australia | 6,000 Packs & 10,000 Packs | - | Apply for registration as it is not a essenscial medicine for WOR. (Not Permitted) |
| 24 | 09-Mar-22 | Tabrane Pharmaceuticals (Pvt) Ltd | LP/DHS/NP/E/013/2019 | Olaparib Capsule 50mg | Drug International Ltd (Unit 2), Bangladesh | 108,80 Capsules (30's) | 650.00 LKR/Capsule | Not Permitted. Register the product. (Not Permitted) |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No./Invoice No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 5-Jan-22 | SPC | DHS/RP/237/20 | Linezolid Tablets 600mg | Glenmark Pharmaceuticals Ltd, India | 31,000 Tablets | 7.73 USD/C&F (5x1x4's) | Previously registered manufacturer, not registered currently. Order has been processed when the manufacturer was registered under Sunhsine Healthcare. Permitted only for this consignment. |
| 2 | 7-Jan-22 | Care Ring Pharma | DHS/P/WW/665/21 | Aprotinin for injection BP 10,000 KIU/Ml, 50 ml Vial | Health Biotech Ltd, India | 50 Vials | 24,500 LKR/Vial | Supplier is not registered with NMRA. Manufacturer is also not registered. Not permitted. |
| 3 | 7-Jan-22 | Hemka Pharma Pvt. Ltd | DHS/RP/NP/O/241/2021 | Vandetanib Tablets 100mg | Kwality Pharmaceuticals, India | 1,080 Tablets | 4,250.00 LKR/Tablet | Supplier is not registered with the NMRA. Manufacturer has had several quality failures. Not permitted. |
| 4 | 11-Feb-22 | SPC/Hemka Pharma Pvt. Ltd | LP/NP/DHS/O/PM/296/2021 | Vendetanib Tablets 100mg | Kwality Pharmaceuticals, India | 1,080 Tablets | 4,250.00 LKR/Tablet | Supplier is not registered with the NMRA. Manufacturer has had several quality failures. Not permitted. |
| 5 | 7-Jan-22 | Care Ring Pharma | DHS/P/WW/177/22 | Testesterone Enathate Injection USP 250mg in 1ml Ampoule | QP Pharma Chem Ltd, India | 650 Ampoules | 958.00 LKR/Vial | Both supplier and manufacturer are not registered with the NMRA. Not permitted |
| 6 | 20-Jan-22 | SPC/Markss HLC Pvt. Ltd | 2021/SPC/O/C/P/00628 | Denosumab Injection 60mg in 1ml | Amgen - UAE/EU | 262 Pre-Filled Syringes | 21,000.00LKR/Pre-Filled Syringe | High priced medicine. Awaiting decision from college. Decision pending |
| 7 | 7-Jan-22 | Markss HLC Pvt. Ltd | LP/NP/DHS/O/MMN/276/2021 | Denosumab Injection 60mg in 1ml | Amgen - UAE/EU | 262 Pre-Filled Syringes | 21,000.00LKR/Pre-Filled Syringe | High priced medicine. Awaiting decision from college. Decision pending |
| 8 | 10-Jan-22 | Care Ring Pharma | DHS/RP/688/2018 | Dimercaprol Injection BP 100mg / 2ml | Arco Life Sciences (India) Pvt. Ltd, India | 20 Ampoules | 49,000 LKR/Ampoule | Not a registered supplier. Cost is also high. See previous WOR minute. Not permitted. (The price in India as INR 340/-. Hence, this price is unjustifiable. Not permitted) |
| 9 | 18-Jan-22 | Talex Pharma | LP/DHS/AMS/3420/2021 | Melatonin Tablets 2mg | Davis Pharmaceuticals Laboratories, Pakistan | 170,000 Tablets | 13.20 LKR/Tablet | WOR granted on 14-02-2022. Not permitted |
| 10 | 18-Jan-22 | SPC/Tabrane Pharmaceuticals | DHS/RP/NP/B/191/2021 (P/O No. LP/NP/DHS/B/PS/235/2021) | Valpatasvir 100mg + Sofosbuvir 400mg Tablets | Drug International Ltd, Bangladesh | 180 Tablets | 990.00 LKR/Tablet | No registered product. Price less than country of origin. Permitted. |
| 11 | 18-Jan-22 | SPC/Tabrane Pharmaceuticals | DHS/RP/NP/B/191/2021 (P/O No. LP/NP/DHS/B/PS/236/2021) | Valpatasvir 100mg + Sofosbuvir 400mg Tablets | Drug International Ltd, Bangladesh | 720 Tablets | 990.00 LKR/Tablet | No registered product. Price less than country of origin. Permitted. |
| 12 | 20-Jan-22 | Tabrane Pharmaceuticals | DHS/RP/CPU/O/090/2020 & DHS/RP/CPU/O/053/2021 | Olaparib Capsules 50mg | Drug International Ltd, Bangladesh | 130,470 + 26,540 Capsules | 269.00 LKR/Capsule | No registered product. Previous WOR no quality issued. Permitted |
| 13 | 28-Jan-22 | SPC/Ceyoka Pvt. Ltd | DHS/PS/106/2021 | Atracuruium Besylate Injection 25mg/2.5ml | Ciron Drugs & Pharamceuticals Pvt Ltd, India | 490 Ampoules | 0.30 USD/Ampoule | Ciron Drugs has had several quality failures. Not permitted. |
| 14 | 12-Jan-22 | SPC/Malachi Holdings Pvt. Ltd | LP/NP/DHS/O/PM/239/2020 | Vandetanib Tablets 100mg | Sanofi Genzyme-UK, USA, Europe | 2,160 Tablets | 12,200.00 LKR/Tablet | Dose is 300mg/day. Cost will be 36,600.00 LKR. 300mg tablets are avialable. Recall after change of specifications. Not permitted. |
| 15 | 12-Jan-22 | SPC/Yaden International | DHS/P/WW/08/22 | Mesalazine Tablets 400mg | Merury Laboraotires, India | 125,000 Tablets | 58.00 LKR/Tablet | Sent to legal department for action against violation of MRP. |
| 16 | 10-Feb-22 | Markss HLC Pvt. Ltd | DHS/RP/140/21 | Lopinavir 100mg + Ritonavir 25mg Tablets | Mylan Laboratories Ltd,India | 303 Packs (120's) | 10,450 LKR/120's Pack | Permitted as no registered product. |
| 17 | 11-Feb-22 | Markss HLC Pvt. Ltd | DHS/P/WW/307/22 | Ritonavir Tablets 100mg | Mylan Laboratories Ltd,India | 50,520 Tablets (30's) | 2,500 LKR/30's | Permitted as WHO PQ. |
| 18 | 10-Feb-22 | Markss HLC Pvt. Ltd | DHS/P/WW/304/2022 | Lamivudine 30mg+Zidovudine 60mg Tablets | Mylan Laboratories Ltd,India | 810 Packs (60's) | LKR 5,500 / 60's | Permitted as there are no registered products. |
| 19 | 12-Feb-22 | Medmart Pharma | DHS/P/WW/318/22 | Pemetrexed for Injection 500mg | Celon Laboratories Pvt. Ltd, India | 350 Vials | 19.00 USD/Vial | Registered products available. Not permitted. |
| 20 | 12-Feb-22 | Pharma Associates | LP/MSD/CPU-DHS/NRQ/151/(T) 2020 | Imiglucerase Injection for Intravenous Infusion 400 IU | ISU Abxis Co. Ltd, Korea | 24 Vials | 248,500 LKR/Vial | There are registered products. Not permitted. |
| 21 | 11-Feb-22 | Truvic Pvt. Ltd | LP/SPC/PA/064/2021 | Loratadine Suspension USP 5mg/5ml | BioPharma Ltd, Bangladesh | 24,000 Bottles (60ml) | 77.25 LKR/60ml Bottle | To check dossier. Decision pending. |
| 22 | 7-Jan-22 | George Steauart Health Pvt. Ltd | EXP/166/21 | Dextran 40 in Sodium Chloride Injection | Thai Otsuka Pharmceutical Co. Ltd, Thailand | 140 Packs | 19.85 USD/Unit price | Registration has not been sought. Local manufactured product registered. Shelflife-remaining shelf life only 5 months. Not permitted. |
| 23 | 31-Jan-22 | A Baur & Co. Ltd | 1242 | Clobazam Tablets IP 10mg | Sanofi India Ltd, India | 10,280 Packs (15x30's) | 25.425 USD/Pack | Pack size change. Permitted. |
| 24 | 5-Jan-22 | A Baur & Co. Ltd | 1242 | Clobazam Tablets IP 10mg | Sanofi India Ltd, India | 10,280 Packs (15x30's) | 25.425 USD/Pack | Pack size change. Permitted. |
| 25 | 12-Feb-22 | A Baur & Co. Ltd | 1242 | Clobazam Tablets IP 10mg | Sanofi India Ltd, India | 10,280 Packs (15x30's) | 25.425 USD/Pack | Pack size change. Permitted. |
| 26 | 26-Jan-22 | Breath Free Lanka | BFL/21/100 | Dutasteride Capsules IP 0.5mg | Cipla Ltd, India | 4,309 Packs (100's) | 17.3 USD/10x10's | Permitted owing to pandemic situation. |
| 27 | 31-Jan-22 | Alaris Lanka Pvt. Ltd | M/3745/NP Request to product registration as a Borderline product | Omega 3 Fish oil soft Gelatin Capsules 750mg | Contract Manufacturing and Packaging Service Pty. Ltd, Australia. | - | - | Rejected by MEC . Not permitted. |
| 28 | 31-Jan-22 | Alaris Lanka Pvt. Ltd | M/2363/NP Appeal for product registration as s borderline | Omega 3 Fish oil soft Gelatin Capsules 1000mg | Contract Manufacturing and Packaging Service Pty. Ltd, Australia. | - | - | Rejected by MEC . Not permitted. |
| 29 | 7-Jan-22 | Alaris Lanka Pvt. Ltd | M/2363/NP request for extention of registration | Omega 3 Fish oil soft Gelatin Capsules 1000mg | Contract Manufacturing and Packaging Service Pty. Ltd, Australia. | - | - | Rejected by MEC . Not permitted. |
| 30 | 18-Feb-22 | SPC | DHS/NV/218/2021 | Sterilized Water for injection BP (SUPERMAP), 10ml Ampoule | Otsuka Pharmaceuticals India Pvt. Ltd, India | 8,800,000 Ampoules | 0.0210 USD/Ampoule | Expedite registration by 22-02-2022 Not permitted. |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No./Invoice No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 7-Dec-21 | SPC | DHS/AMS/352/2021 (Inovoice No. : 90127674186607) | Bivalent Poliomyelitis Vaccine Life 20 dose Vial | Sanofi Pasteur SA-France/Supplier : UNICEF -Denmark | 35,000 Vials (700,000 dose) | 33 USD/Vial | Permitted as it is needed for the EPI program. |
| 2 | 7-Dec-21 | SPC/Yaden International (Pvt) Ltd | DHS/RP/194/2021 | Hydrocortisone Tablets USP 5mg | Mercury Laboratories Ltd, India | 531,250 Tablets (100's) | 15.00 LKR/Tablet | Refer to pricing committee. Product is registered. Not permitted. |
| 3 | 7-Dec-21 | SPC/Care Ring Pharma (Pvt) Ltd | DHS/RP/668/2018 | Dimercaprol Injection BP 100mg/2ml | Arco Lifesciences (India) Ltd, India | 20 Ampoules | 49,000.00 LKR | The price in India as INR 340/-. Hence, this price is unjustifiable. Not Permitted. |
| 4 | 7-Dec-21 | Leamington HLC (Pvt) Ltd | DHS/RP/371/20 (Indent : LP/DHS/AMS/3274/20) | Raltegravir (as Potassium Salt) 400mg Tablets | Merck Sharp & Dohme Crop., Netherland | 22,000 Tablets (1's) | 383.00 LKR /Tablet | Permitted as it is required by STD campaign. BNF price LKR 2,200/-. |
| 5 | 7-Dec-21 | AVH Distributors Pvt. Ltd | DHS/RP/CPU/O/011/21 (Indent : LP/NP/DHS/O/PM/090/21) | Nivolumab INN 100mg/10ml Vial | Becon Pharmaceuticals Ltd, Bangladesh | 274 Vials (1's) | 169,800.00 LKR / Vial | Not come through proper channel. Not Permitted. |
| 6 | 15-Dec-21 | SPC/Ravilux Company Ltd | DHS/RP/657/2018 (Requested for an extention to the WOR. Already granted.) | Morphine Oral Solution BP 0.2% w/v (Morphine Sulphate Oral Solution BP 0.2% w/v) | BDH Industries Ltd, India | 1000 Bottles (100ml) | 18.1 USD/Bottle | Given WOR has expired. Hence, permitted. |
| 7 | 21-Dec-21 | Hemas Pharmaceuticals | SPC/WM/035/2021 (Tender No: RES/21/10/D/2019) | Methylprednisolone Tablets USP 16mg | Pfizer Italia SRL, Italy (Supplier : Pfizer Exports B.V, Netherlands | 6,000 Packs (30's) | 2.36 USD / 3x10 Tablets | P18 please attend. Decision Pending. |
| 8 | 21-Dec-21 | SPC/Malachi Holdings Pvt. Ltd | LP/NP/DHS/O/PM/282/2021 (Tender No: DHS/RP/NP/O/320/21 | Vandetanib Tablets 100mg | Sanofi Genzyme - UK/USA/Europe | 540 Tablets | 12,200.00 LKR/ Tablet | The dose in BNF is 200mg-300mg. The cost/day = LKR 24,400-36,600. The product is not available for the committee to ascertain if it is a genuine product. To get an opinion from college of Oncologist. Decision Pending. |
| 9 | 21-Dec-21 | SPC/Malachi Holdings Pvt. Ltd | LP/NP/DHS/O/PM/167/2021 (Tender No. : DHS/RP/NP/O/184/2021) | Vandetanib Tablets 100mg | Sanofi Genzyme - UK/USA/Europe | 2,730 Tablets (1's) | 12,200.00 LKr/ Tablet | The dose in BNF is 200mg-300mg. The cost/day = LKR 24,400-36,600. The product is not available for the committee to ascertain if it is a genuine product. To get an opinion from college of Oncologist. Decision Pending. |
| 10 | 22-Dec-21 | Citihealth Imports (Pvt) Ltd | HPL/LK/05/2021 (For balance quantity) | Remdesivir Injection 100mg | Healthcare Pharmaceuticals Ltd, Bangladesh | 5000 Packs (1's) | 16.50 USD/Pack | Refer to EUP committee. Decision Pending. |
| 11 | 24-Dec-21 | A Baur & Co. (Pvt) Ltd | 1200011665 dated 02.12.2021 | Methoxy Polyethylene glycol-epoetin Beta Injection 50mcg/0.3ml | F. Hoffmann - la Roche Ltd, Switzerland | 100 Units | 37.52 USD/Unit | Registration is in the process of finalising. Not Permitted. |
| 12 | 24-Dec-21 | SPC/Yaden International (Pvt) Ltd | DHS/RP/193/2021 (2021/SPCN/R/P/00030) | Cefuroxime Sodium Injection BP 250mg | Neon Laboratories Ltd, India (Supplier: Eureka Life Sciences pte. Ltd, Singapore) | 200,000 Vials | 1.30 USD/Vial | Permitted as there is a citical need in the state sector. Registered product has a quality issue and was withheld. |
| 13 | 24-Dec-21 | Talex Pharma (Pvt) Ltd | LP/DHS/AMS/3420/22 (Tender No. : DHS/P/WW/152/22) | Melatonin Tablets 2mg | Davis Pharmaceuticals Laboratories, Pakistan | 170,000 Tablets | 13.20 LKR/Tablet | Permitted. There has been a problem at NMRA regarding classification regarding if it is a borderline product or medicine since 2019. MAHs should be informed to register it is a medicine as it is in the BNF. |
| 14 | 30-Dec-21 | SPC/Harcourts (Pvt) Ltd | DHS/P/WW/548/2016 (Indent : DHS/HD/688/2016) | Gentamycin Sulphate Eye/Ear Drops BP 0.3% w/v | Pliva Pakistan (Pvt) Ltd, Pakistan (Supplier: Royal Group, Pakistan) | 40,000 Bottles (5ml) | 0.117 USD/Bottle | Extend registration. Not Permitted. |
| 15 | 24-Dec-21 | Anti Leprosy Campaign | UCN/NTD/STO | PB Child blisters (PBC), Lamprene 50mg (Clofazimine 50mg), Lamprene 100mg (Clofazimine 100mg), Lamprene 100mg (Clofazimine 100mg), | Donated by World Health Orgaization, Switzerland. | 384 Packs +05 Packs +53 Packs +27 Packs | Free of Charge | |
| 16 | 7-Jan-22 | SPC | DHS/PM/465/21 (DHS/P/DQ/229/2021) | Dextran 40, 10% w/v Sodium Chloride IV use 500ml (Parenteral Solutions) | Thai Otsuka Pharmaceutical Co. Ltd, Thailand. | 760 Bags (Pack size: 1's) | Free of Charge |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 13-Sep-21 | Medmart Pharma (Pvt) Ltd | HB/MPL/210907-01 & HB/MPL/210907-02 | Tocilizumab Injection 400mg/20ml | Chugai Pharmaceuticals Co. Ltd, Japan | 100 packs | LKR 325,000/pack | Permitted on the recommendation of the Secratory, State Ministery of Production, Supply and Regulation of Pharamaceuticals. |
| 2 | 13-Sep-21 | Baur Life Sciences (Pvt) Ltd | BPL/21-22:55 | Remdesivir Injection 100mg/20ml (5mg/ml) | Bharat Parenterals Ltd, India | 50,000 vials | LKR 2,720.00 | Permitted on the recommendation Issued by Secratory, State Ministery of Production, Supply and Regulation of Pharamaceuticals. |
| 3 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/004/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 4 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/011/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 5 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/013/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 6 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/012/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 6000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 7 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/014/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 8 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/015/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 6000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 9 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/002/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 10 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/001/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 11 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/003/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 6000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 12 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/005/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 13 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/006/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 6000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 14 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/007/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 15 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/008/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 16 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/009/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 6000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 17 | 14-Sep-21 | Alaris Lanka (Pvt) Ltd | Alaris/010/21-22 | Paracetamol Tablets BP 500mg | Medopharm - India | 7000 Jar (1000's) | LKR 1.17 | Not permiited as several registered products are avialable. |
| 18 | 14-Sep-21 | A. Baur & Co. (Pvt) Ltd | MLL/BAURS/RDV/POI/27/08/2021 | Remdesivir Injection 100mg/vial | Mylan Laboratories, India | 1000 vials (1's) | LKR 3,653.82 | Permitted on letter of recommendation issued by Secratory, State Minister of Production, Supply & Regulation of Pharmaceuticals. |
| 19 | 16-Sep-21 | Mega Pharma (Pvt) Ltd | ACME/MP-23/21 | Cholecalciferol Tablets 2000 IU | The Acme Laboratories Ltd., Bangladesh | 3,000 packs (3x10's) | LKR 7.78 | Specify (MRP) price for consideration. Decesion Pending. |
| 20 | 16-Sep-21 | ABC Pharma Services (Pvt) Ltd | Invoice not attached | Cholecalciferol Soft Gelatin Capsules 1000IU/200 IU | Zee Laboratories , India | Invoice not attached | LKR 20.00/1000IU & 25.00/2000IU | No invoice attached. Request for same before consideration. Decesion Pending. |
| 21 | 16-Sep-21 | Mega Pharma (Pvt) Ltd | ACME/MP-22/21 | Cholecalciferol Tablets 1000 IU | The Acme Laboratories Ltd., Bangladesh | 3,000 packs (3x10's) | LKR 6.08 | Specify price for consideration. Decesion Pending. |
| 22 | 17-Sep-21 | SPC/Yaden International (Pvt) Ltd | DHS/RP/183/2021 | Phytomenadione Tablets BP 5mg | Mercury Laboratories, India / Supplier : Eureka Life Sciences Pvt. Ltd, Singapore | 112,500 tablets (1,125pkx100's) | LKR 414.26 | Price is very high. Avialable at SPC at LKR 299/- at Union Chemicals at LKR 92/- Not permitted. |
| 23 | 17-Sep-21 | SPC/Pharma Associates | DHS/RP/CPU/O/076/2021 | Tegafur 20mg, Gimeracil and Oteracil Potassium Capsules | Jiangsu Hengrui Medicines Co. Ltd, China | 2,666 Capsules | LKR 1,090.00 | MSD indicates that it was ordered in February and the need may not be relevant now. Not permitted. |
| 24 | 17-Sep-21 | ABC Pharma Services (Pvt) Ltd | ZL/PI/103/2021 | Ivermectin Tablets USP 12mg | Zee Laboratories , India | 10,000 boxes (10x10) | LKR 30.00 | Permitted as within approved price limit & letter of recommendation from the Secretary, State Ministry of Production, Supplies & Regulation of Pharmaceuticals. |
| 25 | 17-Sep-21 | Slim Pharmaceuticals (Pvt) Ltd | CH/EXP-PI/2122/080 | Ivermectin Tablets USP 12mg | Cian Healthcare Ltd, India | 20,000 boxes (10x10) | LKR 4.05 (CIF) | Specify price (MRP) for consideration. Decesion Pending. |
| 26 | 17-Sep-21 | Baur Life Sciences (Pvt) Ltd | BPL/21-22:58 | Ivermectin Tablets USP 12mg | Bharat Parenterals Ltd, India | 35,000 Packs (3x10's) | LKR 2.06 (FOB) | Specify Price (MRP) for consideration. Decesion Pending. |
| 27 | 17-Sep-21 | Tropical Pharma Pvt. Ltd | TROPICAL-02/2021 | Ivermectin Tablets USP 6mg | Drug International Pvt. Ltd, Bangladesh | 10,000 packs (10's) | LKR 15.42 (FOB) | Specify Price (MRP) for consideration. Decesion Pending. |
| 28 | 17-Sep-21 | Trimed Pharma Pvt. Ltd | F&R-PI-21-45 | Ivermectin Tablets 3mg | Focus & Rulz Pharmaceuticals (Pvt) Ltd, Pakistan | 10,000 packs (1x10's) | LKR 151.83 (FOB) | Specify price (MRP) for consideration. Decesion Pending. |
| 29 | 17-Sep-21 | Trimed Pharma Pvt. Ltd | F&R-PI-21-45 | Ivermectin Tablets 6mg | Focus & Rulz Pharmaceuticals (Pvt) Ltd, Pakistan | 10,000 packs (1x10's) | LKR 174.16 (FOB) | Specify price (MRP) for consideration. Decesion Pending. |
| 30 | 17-Sep-21 | Trimed Pharma Pvt. Ltd | F&R-PI-21-45 | Ivermectin Tablets 12mg | Focus & Rulz Pharmaceuticals (Pvt) Ltd, Pakistan | 10,000 packs (1x10's) | LKR 196.49 (FOB) | Specify price (MRP) for consideration. Decesion Pending. |
| 31 | 17-Sep-21 | CIC Holdings PLC | PI-SL-CIC-08-21 | Ivermectin Tablets 6mg | Advanced Chemical Industries Ltd, Bangaldesh | 100,000 packs (10's) | LKR 13.19 (CIF) | Specify price (MRP) for consideration. Decesion Pending. |
| 32 | 17-Sep-21 | Hemas Pharmaceuticals (Pvt) Ltd | GHPL/RI/21-22/001 | Ivermectin Tablets 3mg , 6mg, 12mg | Gentech Healthcare Pvt. Ltd, India | 2000 boxes in each strength | 3 mg - LKR 2.80 / 6 mg -LKR 3.36 / 12 mg - LKR 6.33 | New manufacturer. Price also not clear. Decision at next meeting after all documents are submitted. Decesion Pending. |
| 33 | 17-Sep-21 | Cliniqon Biotech Pvt. Ltd | PFI/ICL/2001/019/2021-22 | Ivermectin Dispersible Tablets 12mg | Innova Captab Ltd, India | 5000 packs (10x10) | LKR 29.50 | Permitted as documents have been submitted & evaluated as acceptable. |
| 34 | 17-Sep-21 | Sunway Healthcare Pvt. Ltd | KTPL/047/21-22 | Ascorbic acid tablets 500mg (Vit. C) | Kausikh Therapeutics (P) Ltd, India | 10,000 (10x10) | LKR 3.34 (CIF) | Not permitted as Vit. C is not an emergency medicine. |
| 35 | 23-Sep-21 | SPC | DHS/P/WW/549/21 | Alfentanil Hydrochloride Injection 1000 mcg in 2ml amp | Siegfried Hameln GmbH, Germany (Supplier : Hameln Pharma GmbH, Germany) | 300 amp | LKR 3,453.80 (FOB) | Alfentanil has not available for the state sector since 2017. Price is high. Hence, it was not permitted as it is not an essential medicine. |
| 36 | 24-Sep-21 | Sunshine Healthcare Lanka Ltd | Invoice No.58 | Remdesivir for Injection 100mg/vial | Cadila Healthcare Ltd, India | 2000 vials | LKR 4,871.76 (CIF) | Specify MRP for consideration. Decesion Pending. |
| 37 | 27-Sep-21 | Markss HLC (Pvt) Ltd | Invoice not attached | Remdesivir 100mg Injection | Mylan Laboratories, India | 10,000 vials | - | Required documents not submitted. Not permitted. |
| 38 | 27-Sep-21 | Barck International (Pvt) Ltd | ECEX-058 | Ascorbic acid tablets 500mg (Vit. C) | Emmchak Formulations (Pvt) Ltd, India | 996 pcks (10x10's) | LKR 4.05 (CIF) | Not permitted as not an emergency use medication. |
| 39 | 28-Sep-21 | SPC/Srag Biotech Ceylon (Pvt) Ltd | SPC/WM/038/2020 | Hydrocortisone Acetate Ophthalmic Ointment 1% | Ambalal Sarabhai - India (Supplier : Asence Pharma Pvt. Ltd) | 30,000 tubes (3.5g) | LKR 58.46 | Expedite registration. Not permitted. |
| 40 | 29-Sep-21 | Markss HLC (Pvt) Ltd | LP/NP/DHS/B/MMN/012/2019 | Anagrelide (as hydrochloride) Capsules 500mcg | Haupt Pharma, Germany | 990 caps | LKR 761.00 | To submit PIL to check if it is in English. Also, re-inform to register with NMRA. Decesion Pending. |
| 41 | 29-Sep-21 | Malachi Holdings (Pvt) Ltd | LP/NP/DHS/O/PM/167/21 | Vandetanib Capsules 100mg | Sanofi Genzyme-UK, USA, Europe | 2,730 Caps | LKR 12,200.00 | To register under orphan drug. Not permitted. |
| 42 | 29-Sep-21 | Care Ring Pharma (Pvt) Ltd | LP/NP/DHS/O/PM/187/21 | Nivolumab Injection 100mg / 10ml | Bristol Myersa Squibb, Turkey | 36 Vials | LKR 147,000.00 | Not permitted after considering reliability, quality failure. |
| 43 | 29-Sep-21 | Care Ring Pharma (Pvt) Ltd | LP/NP/DHS/O/PM/190/21 | Nivolumab Injection 100mg / 10ml | Bristol Myersa Squibb, Turkey | 36 Vials | LKR 152,500.00 | Not permitted as not reliable & also quality failure. |
| 44 | 29-Sep-21 | Care Ring Pharma (Pvt) Ltd | LP/NP/DHS/O/PM/189/21 | Nivolumab Injection 100mg / 10ml | Bristol Myersa Squibb, Turkey | 36 Vials | LKR 152,500.00 | Not permitted for the following after considering reliability & previous supply & final price. |
| 45 | 30-Sep-21 | Tabrane Pharmaceuticals (Pvt) Ltd | DHS/RP/CPU/O/090/2020 | Olaparib Capsule 50mg | Drug International Pvt. Ltd, Bangladesh | 130,470 cap | LKR 269.00 | Not permitted as given at previous WOR. |
| 46 | 4-Oct-21 | SMM Halcyon (Pvt) Ltd | SPC/NN/016/2021 | Vitamin E Capsules UPS 400mg | XL Laboratories Ltd, India | 120,000 packs (10x10's) | LKR 3.24 | Expedite site change docs. Not permitted. |
| 47 | 4-Oct-21 | Hemka Pharma (Pvt) Ltd | DHS/RP/158/2021 | Danazole Capsules 100mg | Slaus Pharmaceuticals, India | 120,000 Cap | LKR 31.50 | Registered products available. Not permitted. |
| 48 | 4-Oct-21 | Sri Lanka Cricket | Donation of Bangladesh Cricket Board | (i) Actemra 400mg (ii) Ivermectin 12mg | No details attached | (i) 15 vials (ii) 6000 tablets | No Invoice | Inadequate documentation. Not permitted. |
| 49 | 6-Oct-21 | A Mart Holdings (Pvt) Ltd | BD0321/SRI | Tocilizumab Injection 400mg | Roche-Japan | 10 vials | LKR 295,000.00 (Not MRP) | Permitted on the letter of recommendation forwarded by Secretary, State Ministry of Production, Supply and Regulation of pharmaceuticals |
| 50 | 6-Oct-21 | Markss HLC (Pvt) Ltd | DHS/RP/NP/B/236/2021 | Cinacalcet Tablets 30mg | M/S Nobel - Turkey | 540 nos | LKR 750.00 | Register product. Not permitted. |
| 51 | 6-Oct-21 | Markss HLC (Pvt) Ltd | DHS/RP/NP/O/238/2021 | Denosumab Injection 60mg in 1ml | M/S Amgen - USA, Europe | 226 PFSY | LKR 19,000.00 | Need letters of justification. Not permitted. |
| 52 | 6-Oct-21 | George Steaurt Health (Pvt) Ltd | HB/83498/SR/21 | Ivermectin Tablets USP 12mg | Taj Pharmaceuticals Ltd, India | 20,000 packs (10x10's) | LKR 30.00 (MRP) | Permitted as within MRP range. |
| 53 | 6-Oct-21 | Pharma Associates | PF 024 | Ivermectin 12mg tablets | Sano-Cito Therapeutics Inc., India | 5,000 packs (10x10's) | LKR 15.00 (MRP) | Not a registered manufacturer. Sufficient product have been approved. Hence, not permitted. |
| 54 | 6-Oct-21 | Cloud Healthcare (Pvt) Ltd | Inv. 052 | Ivermectin 3mg tablets | Laborate Pharmaceuticals, India | 10,000 packs (10x10's) | LKR 5.48 (FOB) | Not permitted as it is not approved for paediatrics use. |
| 55 | 6-Oct-21 | Emar Pharma (Pvt) Ltd | WPPL/30/2021-22 | Ivermectin Tablets 12mg | Mascot Health Series Pvt. Ltd, India | 275,000 tablts | LKR 7.10 (CIF) | Specify Price (MRP) for consideration. Decesion Pending |
| 56 | 28-Sep-21 | National STD/AIDS Control Programme | SI3621223156 | Tenofovir Disoproxil Fumarate 300mg + Emitricit bine 200mg | Hetero Labs Ltd, India | 28,080 tablets (30's) | - | Donation (Free of charge) |
| 57 | 27-Sep-21 | UNFPA Country Office, Sri Lanka | 813A121469 | Levonogrestrel Subdermal Implants 75mg (Jadelle Implants 75mg) | Bayer AG, Germany | 2x75mg, 100xpouch UNFPA | - | Donation (Free of charge) |
| 58 | 8-Oct-21 | Hemophilia Association | #636 | Listed items | WFH (World Federation of Hemophilia) | Listed qty | - | Donation (Free of charge) |
| 59 | 15-Oct-21 | Anti TB Drugs | 21210752 SO | Listed items | IDA Foundation, The Netherlands | Listed qty | - | Donation (Free of charge) |
| S. No. | Date of issue | Institute | Name of Medicine | Qty | Manufacturer/Donator | Decision |
| 1 | 2-Sep-21 | Slim Pharmaceuticals | Casirivimab & Imdevimab Concentrate for Solution for infusion 1333mg/11.1ml | 1,250 vials | F. Hoffmann La-Roche Ltf-Switzerland | Issued WOR as decided by Chairman (NMRA), CEO (NMRA), Secretary,(MEC) & Regulatory Pharmacist (NMRA) |
| 2 | 2-Sep-21 | Slim Pharmaceuticals | Casirivimab & Imdevimab Concentrate for Solution for infusion 300mgg/2.5ml | 5,000 vials | F. Hoffmann La-Roche Ltf-Switzerland | Issued WOR as decided by Chairman/NMRA, CEO/NMRA, Secretary/MEC & Regulatory Pharmacist/NMRA |
| 3 | 9-Sep-21 | Innogen Lanka | Ivermectin 12mg Tablet | 10,000 packs (10x10's) | Mc. W. Healthcare (Pvt) Ltd-India | Permitted for this consigment. |
| 4 | 31-Aug-21 | ABC Pharma (Pvt) Ltd | Ivermectin Tablets 6mg | 100,000 pkts. | Popular Pharmaceuticals Ltd, Bangladesh | Permitted under emergency use as there is some limited evidence of efficancy in Covid-19 |
| 5 | 8-Sep-21 | Emerchemie NB (Ceylon) Ltd | Ivermectin Tablets 6mg & 12 mg | 2,500 & 25,000 packs | Beximco Pharmaceuticals Ltd-Bangladesh | Permitted for this consigment. |
| 6 | 17-Aug-21 | George Steuart | Ivermectin Tablets USP 12mg | 10,000 pkt (10x10's) | Taj Pharmaceuticals, India | Emergency Use Permission is granted as there is limited evidence that it is effective for patient with COVID 19 |
| 7 | 8-Sep-21 | George Steaurt | Ivermectin Tablets USP 12mg | 9,985 Packs | Taj Phrmaceuticals Ltd-India | Permitted for this consigment |
| 8 | 16-Sep-21 | Villogen Pharma (Pvt) Ltd | Ivermectin USP 12mg | 10,000 tablets | Medicraft Pharmaceuticals (Pvt) Ltd-Pakistan | Permitted |
| 9 | 17-Sep-21 | NHSL - Sri Lanka | Plasma Iyte a solution for infusion 500ml | 20 (20EA/CA) | Baxter India Pvt Ltd-India | FOC-For research purpose |
| 10 | 31-Aug-21 | George Steuart | Remdesivir for Injection 100mg/vial (5mg/ml) | 500 vials | Aspiro Pharma Ltd, India | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 11 | 1-Sep-21 | Emerchemie NB (Ceylon) Ltd | Remdisivir 100mg Injection | 250 box (1's) | Beacon Medicare Ltd-Bangladesh | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 12 | 1-Sep-21 | Breathfree Lanka | Remdisivir for Injection 100mg/vial | 2,040 vials | Cipla Ltd-India | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 13 | 1-Sep-21 | The Esses Pharmacy (Pvt) Ltd | Remdisivir for Injection 100mg/vial | 2,000 vials | Bruck Pharma-India | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 14 | 3-Sep-21 | George Steaurt | Remdisivir for Injection 100mg/vial | 1,000 vials | Hetero Labs-India | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 15 | 3-Sep-21 | George Steaurt | Remdisivir for Injection 100mg/vial | 1,000 vials | Hetero Labs-India | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 16 | 1-Sep-21 | ABC Pharma | Remdisivir Injection 100mg | 30,000 pkt | Popular Pharmaceuticals - Bangladesh | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 17 | 8-Sep-21 | Citihealth Imports | Remdisivir Injection 100mg | 5,000 packs | Healthcare Pharmaceuticals-Bangladesh | Issue WOR as instructed by Hon. Minister (PSRP) |
| 18 | 8-Sep-21 | Citihealth Imports | Remdisivir Injection 100mg | 5,000 packs | Incepta Pharmaceuticals-Bangladesh | Issue WOR as instructed by Hon. Minister (PSRP) |
| 19 | 8-Sep-21 | Hemas Pharmaceuticals | Remdisivir Lyophilized Powder for Injection 100mg/vial | 3,000 vials | Mylan Laboratories Ltd-India | Permitted but, should market under guideline. |
| 20 | 2-Sep-21 | Tabrane Pharmaceuticals | Remdisivir Lyophilized Powder for IV Infusion | 100 vials | Drug International Ltd-Bangladesh | As per the decision taken at the meeting held on 31-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 21 | 8-Sep-21 | Pharma Associates | Tocilizumab 400mg/20 ml Solution for infusion | 50 vials | Roche Manufacturin Co. Ltd, Japan | As per the decision taken at the meeting held on 26-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 22 | 31-Aug-21 | The Esses Pharmacy Pvt. Ltd | Tocilizumab 400mg/20ml Inj. & Tocilizumab 200mg/20ml Inj. | 100 & 100 | Roche- Germany | As per the decision taken at the meeting held on 26-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 23 | 7-Sep-21 | Cloud Healthcare (Pvt) Ltd | Tocilizumab 400mg/20ml vials | 2,000 vials | Roche Manufacturin Co. Ltd, Japan | As per the decision taken at the meeting held on 26-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 24 | 7-Sep-21 | Glah Lanka Pvt. Ltd | Tocilizumab Injection 400mg/20ml | 500 vials | Roche-Switzerland | Issued a WOR as instructed by Hon. State Minister of PSRP. |
| 25 | 2-Sep-21 | Pharma Associates | Tocilizumab Injection 400mg/20ml Solution for infusion | 100 vials | Roche Manufacturin Co. Ltd, Japan | As per the decision taken at the meeting held on 26-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 26 | 20-Aug-21 | Yaden International (Pvt) Ltd | Tocilizumab Solution for Infusion 400mg/20ml & 200mg/20ml | 200 vials each strength | Roche Registration GmbH, Germany | As per the decision taken at the meeting held on 26-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| 27 | 2-Sep-21 | Pharma Associates | Tocilizumab 400mg/20 ml Solution for infusion | 100 vials | Roche Manufacturin Co. Ltd, Japan | As per the decision taken at the meeting held on 26-08-2021 at the office of the State Minister of PSRP with Hon. State Minister of PSRP, Secretary to the State Ministry of PSRP, Chairman/NMRA, CEO/NMRA and the Regulatory Pharmacist of WOR Committee of NMRA decided to grant WOR for import and customs clearance of consigment. |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 3-Aug-21 | A. Mart Holdings (Pvt) Ltd | DHS/P/WW/CPU/B/02/2020 | Vedolizumab Powder for Injection 300mg | Takeda - Japan | 7 vials | LKR 318,000.00 | No details of patient or justification by consultant is included. Not permitted. |
| 2 | 3-Aug-21 | Tabrane Pharmaceuticals (Pvt) Ltd | DHS/RP/CPU/O/047/2021 | Olaparib Capsule 50mg | Drug International Ltd-Bangladesh | 29,300 capsules | LKR 260.00 | 1). No in Bangladesh Drug Regulatory authority. 2). Not in Drug International -Bangladesh web site. 3). Certificate of Pharmaceutical Product (COPP) provided is not orginal. Should submit original COPP. Not Permitted. |
| 3 | 4-Aug-21 | Orexo Bio Parma (Pvt) Ltd | DHS/P/WW/28/21 | Cyclophosphamide Tablet BP/USP 50mg | Health Biotech Ltd, India | 300,000 tablets | LKR 29.00 | Permitted only for this. Orexo Bio Pharma Ltd should immediately send the documents for registration. No WOR will be considered after this. |
| 4 | 4-Aug-21 | Markss HLC (Pvt) Ltd | DHS/P/WW/393/18 | Cholecalciferol Tablet 400IU | MMC Healthcare - India | 375,000 tablets | LKR 2.45 | Medical Supplies Division revision deleted the product. Not Permitted. |
| 5 | 5-Aug-21 | SPC-Markss HLC (Pvt) Ltd | LP/DHS/MM/3270/2021 | Ritonavir Tablet 100mg | Mylan Laboratories Ltd-India | 43,740 tablets | LKR 86.33 | Permitted only for this import. Should register for future. Priority to be given. |
| 6 | 5-Aug-21 | SPC/Mansel (Ceylon) Pvt. Ltd | 2021/SPC/N/C/P/00125 | Dolutegravir Tablet 50mg | Hetero Ltd-India | 243,000 tabs | No attached details | Permitted. Should register for future. |
| 7 | 6-Aug-21 | SPC & Yaden International (Pvt) Ltd | DHS/P/WW/545/21 | Fentanyl Transdermal Patches 12.5mcg/h | Rusan Parma Ltd-India | 4,000 patches | LKR 1,390.00 | Deleted in Medical Supplies Division list. Not permitted. |
| 8 | 6-Aug-21 | Yaden International (Pvt) Ltd | DHS/RP/257/2020 | Lidocaine Injection BP 2% w/v | Mercury Laboratories - India | 10,000 vials | Purchase order/Indent not provided. | This product is manaufactured in India. Supplier is from Singapore. Yaden is registered as Marcket Authorization Holder in Sri Lanka. Not permitted. Supply direct from India. |
| 9 | 12-Aug-21 | Sunshine Healthcare Ltd | LP/P/CPU/DHS/O/E/154/2020 | Eribulin Mesylate Injection 1mg/2ml vial | Natco Parma Ltd-India | 80 vials | LKR 49,000.00 | Not needed by Medical Supplies Division. Not permitted. |
| 10 | 12-Aug-21 | Ceyoka Pvt. Ltd | DHS/RP/127/2021 | Pneumococcal Polysacchride Vaccine | Chengdu Institute of Biological Product Co. Ltd-China | 15,000 vials | LKR 4,000.00 | Referred to external expert for opinion. |
| 11 | 12-Aug-21 | Gulf Pharma | LP/NP/DHS/O/PM/102/21 | Cabazitaxel Injection 60mg/1.5 ml with Solvent | BDR Pharmaceuticals International - India | 18 vials | LKR 30,340.00 | Need to check if patients' need from oncologist. Not permitted. |
| 12 | 16-Aug-21 | A. Baur & Co. Ltd | Invoice No-1237 dated 27.07.2021 | Clobazam Tablets IP 10mg | Sanofi India Ltd- India | 10,280 Tablets | LKR 33.23 | Permitted for this consigment. |
| 13 | 18-Aug-21 | A. Baur & Co. Ltd | LP/NP/DHS/O/SA/138/21 | Eribulin Mesylate Solution for Injection 0.5mg/ml | BSP Pharmaceuticals S.P.A-Italy | 164 vials | LKR 81,000.00 | LKR 81,000 is the agreed price. Hence, permitted for the tender. |
| 14 | 18-Aug-21 | A. Baur & Co. Ltd | LP/NP/DHS/B/E/212/2020 | Fingolimod capsules 500mcg | Novartis Parma Stein AG-Switzerland | 3,584 capsules | LKR 4,463.21 | Permitted as this product has completed the evaluation process but owing to the deletion of the database it is not recoverable. |
| 15 | 18-Aug-21 | A. Baur & Co. Ltd | LP/NP/DHS/B/E/084/2021 | Fingolimod capsules 500mcg | Novartis Parma Stein AG-Switzerland | 1,092 capsules | LKR 4,463.21 | Permitted as product evaluated, price determined & ready for registration. |
| 16 | 18-Aug-21 | SPC-Farnell Ceylon (Pvt) Ltd | SPC/KDT/060/2020 | Albendazole Tablets USP 400mg | Lark Laboratories (India) Ltd-India | 17,500 packs of 8x3's | LKR 4.77 | Permitted for this consigment. Extend registration. |
| 17 | 18-Aug-21 | A. Baur & Co. Ltd | LP/NP/DHS/B/E/097/2021 | Fingolimod capsules 500mcg | Novartis Parma Stein AG-Switzerland | 1,092 capsules | LKR 4,463.21 | Permitted as dossiers are evaluated and price approved. |
| 18 | 20-Aug-21 | National Programme for Tuberculosis Control and Chest Diseases | 39363 | Listed drugs | IDA Foundation | as per the invoice | - | Permitted with free of charge (from IDA Foundation) |
| 19 | 20-Aug-21 | A. Baur & Co. Ltd | DAMPS/TENDER/2021/62 | Methoxy polyethylene glycol epoetin injection 100mcg/0.3 vial / prefilled syringe | F. Hoffmann - La Roche Ltd-Switzerland | 680 vials | LKR 12,897.15 | Permitted as the delay in registration procedure. |
| 20 | 20-Aug-21 | A. Baur & Co. Ltd | DAMPS/TENDER/2021/62 | Methoxy polyethylene glycol epoetin injection 50 mcg/0.3 vial / prefilled syringe | F. Hoffmann - La Roche Ltd-Switzerland | 55 vials | LKR 7,456.16 | Permitted for Army Tender. |
| 21 | 20-Aug-21 | A Mart Holdings | LP/NP/DHS/B/SP/137/2021 | Omalizumab Injection 150mg/ml 1ml Pre Filled Syringe | Novartis - UAE | 218 Pre Filled Syringers | LKR 36,880.00 | Product will be registered soon. Buy from registered Market Authorization Holder. Not permitted. |
| 22 | 20-Aug-21 | Gulf Pharma | LP/NP/DHS/O/PM/129/21 | Cladribine Injection 10mg/10ml | Fresenius Kabi Oncology Ltd-India | 24 Vials | LKR 37,999.00 | Negotiate for LKR 35,000.00 Not permitted. |
| 23 | 24-Aug-21 | SPC | LP/CPU-DHS/308/2020 | Imiglucerase Injection 400 IU/ 10 ml vial | Malachi Holdings (Pvt) Ltd | 60 vials | - | Registered on 06/03/2021. Buy from registered source. Not permitted. |
| 24 | 31-Aug-21 | Care Ring Pharma (Pvt) Ltd | DHS/RP/443/20 | Crotamitone Cream 10%, 20g Tube | Anphar Organics Pvt. Ltd - India | 5,000 tubes | LKR 650.00 | Registered product available. Manufacturer & Supplier not registered with NMRA. Not permitted. |
| 25 | 31-Aug-21 | Care Ring Pharma (Pvt) Ltd | LP/NP/DHS/O/P/M/237/20 | Nivolumab Injection 100mg/10ml | Bristol Myers Squibb - Turkey | 52 vials | LKR 129.96 | Suppliers not registered. Recently order placed by Medical Supplies Division. Hence, not permitted. |
| 26 | 31-Aug-21 | B Braun Lanka Pvt. Ltd. | Inv. 1807847147 | Sterile Water for Injection EP 500ml | B. Braun Medical Industries Sdn. Bhd-Malaysia | 26,000 bottles | LKR 125.87 | Not permitrted. Discuss with Kalun Life Sciences to Manufacture locally. |
| 27 | 9-Sep-21 | Hemas Pharmaceuticals | PI/2122/135 | Favipiraivin Lyophilized powder for Injection 100mg/ml | Bajaj Healthcare Ltd-India | 50,000 packs (1x10's) | LKR 40.97 | Check with Covid committee. |
| 28 | 7-Sep-21 | Markss HLC (Pvt) Ltd | Ref. 06/09/2021/005 | Remdesivir 100mg Injection | Mylan Laboratories Ltd-India | 10,000 vials | - | Not approved. Indicated by Ministry to give WOR. Price negotiated at the Ministry. Invoice not submitted. |
| 29 | 9-Sep-21 | SPC | DHS/C/MM/368/2020 | Tenecteplase Injection 50mg vial | Boehringer Ingelheim Pharma- Germany | 2,000 vials | LKR 89,693.96 | Permitted for this consigment to tide over shortage. They are giving it at the same price negotiated by NMRA. |
| 30 | 9-Sep-21 | SPC/Orexo Bio Pharma Pvt. Ltd | LP/DHS/IG/3102/21 | Cyclophosphamide Tablet BP/USP 50mg | Health Biotech Ltd, India | 300,000 tablets | LKR 29.00 | This is not permitted as WOR was given for another consigment from same manufacturer & Orexo Bio Pharma Pvt. Ltd. |
| 31 | 9-Sep-21 | Sunshine Healthcare Ltd | NSHEX0221500042 | Adapalene 1mg & Benzoyl Peroxide 25mg Gel. (M.F site change) | Encube Ethicals Pvt. Ltd, India for Galderma India Pvt. Ltd | 15,000 tubes | - | Permitted. |
| 32 | 6-Sep-21 | Villogen Pharma (Pvt) Ltd | Invoice No. SRI/03/2021 | Ivermectin USP 12mg | Medicraft Pharmaceuticals (Pvt) Ltd, Pakistan | 10,000 tablets | LKR 19.50 | Permitted. |
| 33 | 7-Sep-21 | Hemas Pharmaceuticals | Ref. 27/08/2021/0013 | Remdesivir Lyophilized Powder for Injection 100mg | Mylan Laboratories Ltd-India | 3,000 vials | LKR 5,689.00 | Permitted but should market under guideline. |
| 34 | 3-Sep-21 | Medmart Pharma (Pvt) Ltd | Invoice No. 20043789 | Itolizumab For Injection (r-DNA Origin) 100mg/vial | Biocon Biologics India Ltd, India | 20 vials | To get opinion from Covid-19 committee. | |
| 35 | 9-Sep-21 | Niix Holdings (Pvt) Ltd | Invoice No- KE 1270 | Atracurium Besylate 10mg/ml Injection | Joint Stock Company - Latvia | 50,000 ampoules | LKR 550.00 | Permitted as registered products are not available till November. Verbally checked over phone. Should register for future suppliers. |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 25.06.2021 | Markss HLC (Pvt) Ltd | DHS/RP/CPU/B/025/2020 | Anagrelide (As Hydrochloride) Capsule 500 mcg | Haupt Pharma-Germany | 720 Capsules | LKR 684.00 | Negotiated to LKR 674/=. Permitted as price is below BNF. However the product is from Germany & there is no information of the language of SmPC. Approved if it is in English. Future supply product has to be registered by Markss . |
| 2 | 25.06.2021 | Markss Healthcare | DHS/P/WW/729/21 | Abacavir Sulphate 60 mg + Lamivudine 30 mg Tablets | Mylan Laboratories Ltd-India | 48,600 Tablets | LKR 102.50 | Stability data Ceritificate of Analysis & Certificate of Pharmaceutical Product have been submitted. Hence this can be permitted . CEO/NMRA to explore a mechanism via orphan medicines to register. Price negotiate to LKR 101/= |
| 3 | 25.06.2021 | Markss HLC Pvt Ltd | DHS/RP/CPU/O/041/2021 | Cladribine Injection 10 mg/10 ml | Fresenius Kabi India Pvt Lts-India | 25 Vials | LKR 38,000.00 | No letter of justification from end-user. |
| 4 | 29.06.2021 | Euro Chem Bio tech (Pvt) Ltd | DHS/RP/CPU/O/039/2021 | Vendetanib Tablets 100 mg | Kwality Pharmaceuticals -India | 2,175 Tablets | LKR 4,900.00 | Not Permitted. All are old requests from 2020 December 2021 January .New letters of justification is needed for each patients . |
| 5 | 29.06.2021 | Euro Chem Bio tech (Pvt) Ltd | DHS/RP/CPU/O/060/2021 | Vendetanib Tablets 100 mg | Kwality Pharmaceuticals -India | 900 Tablets | LKR 4,900.00 | Not Permitted . All are old requests from 2020 December & 2021 January .New letters of justification is needed for each patients . |
| 6 | 02.06.2021 | SPC | LP/MSD/CPU-DHS/NRQ/069/(T)/2020 | Tretinoin Capsule 10 mg | 5,260 Capsules | Not Permitted due to Registered products are available . | ||
| 7 | 29.06.2021 | Care Ring Pharma (Pvt) Ltd | DHS/P/WW/755/21 | Glycopyronium Bromide Tablets 1 mg | Cotec Healthcare (Pvt) Ltd- India | 5,000 Tablets | LKR 197.00 | 1) MSD informed that not essential. 2) Care Ring Pharma & manufacturer not registered. Therefore not permitted. |
| 8 | 30.06.2021 | Markss Healthcare Pvt Ltd | DHS/RP/146/20 | Efavirenz Tablets 200 mg | Mylan Lboratories Ltd - India | 18,000 Tablets | LKR 166.50 | Not Permitted. SPC informs that it is cancelled. |
| 9 | 30.06.2021 | Malachi Holdings Pvt Ltd | DHS/RP/CPU/0/073/2021 | Denosumab Injection 60 mg in 1 ml | Amgen -USA/EU | 270 PFSY | LKR 22000.00 | Not Permitted. No letter of justification from end-user. |
| 10 | 09.07.2021 | A MART Holdings (Pvt) Ltd | DHS/RP/CPU/0/104/2020 | Denosumab Injection 60 mg in 1 ml | Amgen -USA/EU | 272 PFSY | LKR 18380.00 | Not Permitted. No letter of justification from end-user. |
| 11 | 30.06.2021 | SPC / Yaden International Pvt Ltd | DHS/RP/257/20 | Lignocaine Injection 2% 5ml vial | Mercury Laboratories -India / Eureka Life Sciences Pte Ltd -Singapore | 10,000 Vials | LKR 1336.44 | Not permitted. MSD/SPC indicates not needed. |
| 12 | 30.06.2021 | Yaden International Pvt Ltd | LP-P-CPU/DHS/O/P/M/027/2021 | Docetaxel I njection 80mg/8ml & 20mg/2ml | Biozenta Lifesciences Pvt Ltd-India | 277 vials of 80mg/8ml vial & 348 vials of 20mg/2 ml vial | LKR 15700/ & 14800/ per vial | Not Permitted. Registered products availble . |
| 13 | 30.06.2021 | Leader Parma Agency Pvt Ltd | DHS/P/DQ/659/2018 | Varecella- Zoster Immunoglobulin 250 mg solution for injection | Masters Pharmaceuticals Ltd -UK / Bio Products Laboratories Ltd (BPL) -UK | 90 Ampoules | LKR 258,035.82 | Permitted as needed in emergency. Negotiate the price. |
| 14 | 07.07.2021 | Care Ring Pharma (Pvt) Ltd | DHS/RP/84/2021 | Tetrabenazine Tablets 25 mg | Cotec Healthcare Pvt Ltd | 75,000 Tablets | LKR 66.65 | Rejected as both supplier & manufacturer are not registered . |
| 15 | 07.07.2021 | Sunshine Healthcare Pvt Ltd | DHS/ NV /583/2020 | Carboplatin Injection BP 450 mg/45 ml | Adley Formulation Pvt Ltd -India | 11,000 Vials | LKR 2,761.63 | Not permitted and advised NMRA to Extend registration. |
| 16 | 07.07.2021 | R & N Vision Pharma | DHS/RP/CPU/0/093/2021 | Mitotaine Tablets 500 mg | Thedose Pharama Pvt Ltd - India | 2,880 Tablets | LKR 1,290.00 | Permitetted for this consignment . Should register for future as both manufacturer & local agent are registered with NMRA. |
| 17 | 21.06.2021 | A MART Holdings (Pvt) Ltd | DHS/RP/CPU/0/100/2021 | Nivolumab Injection 100mg/10ml vial | Bristol Myer Suibb -USA | 176 Vials | LKR 157,974.00 | Need individual letters of justification. |
| 18 | 07.07.2021 | Inventis Pharma (Pvt) Ltd | DHS/RP/CPU/0/018/2021 | Nivolumab Injection 100mg /10 ml vial | Bristol Myers Suibb India (Pvt) Ltd -Puertorico | 52 Vial | LKR 168,750. 00 | Need individual letters of justification. |
| 19 | 07.07.2021 | A.V.H Distributors (Pvt) Ltd | LP/NP/DHS/0/PM/090/2021 | Nivolumab Injection 100mg /10 ml vial | Becon Pharmaceuticals Limited - Bangladesh | 274 Vial | LKR 169,800. 00 | Need individual letters of justification. |
| 20 | 22.07.2021 | SPC/Malachi Holding Pvt Ltd | LP/MSD/CPU-DHS/NRQ/310/T/2020 | Nivolumab Injection 100mg /10 ml vial | Bristol-Myers Squibb -USA | 52 Vials | LKR 160,000.00 | For H.P. Jayasooriya 52 authorised. 25 vials issued. Patient is surviving balance 27 vials permitted. |
| 21 | 07.07.2021 | Gulf Parma Pvt Ltd | DHS/RP/CPU/0/099/2021 | Carmustine for injection IP 100 mg | Celon Laboratoroes Pvt Ltd - India | 9 Vials | LKR 15,500.00 | Not permitted .No letters of justification from end users. |
| 22 | 07.07.2021 | Eureka Life Sciences - Singapore | DHS/P/WW/712/21 | Albendazole Syrup 200 mg 5 ml,30 ml | Mercury Laboratories -India | 2500 Bottles | LKR 6,159.04 | Not permitted. Cost very high. chewable tablets are availble. |
| 23 | 22.07.2021 | Slim Pharmaceuticals | DHS/PM/395/2021 | Albendazole Tablets 400 mg | J.Duncan Healthcare Pvt Ltd - India | 75,000 Tablets | Registered products are availbale therefore Not permitted. | |
| 24 | 07.07.2021 | SMM Halcyon (Pvt) Ltd | DHS/RP/CPU/B/067/2021 | HepBest (Tenofovir Alafenamide Tablets 25 mg) | Mylan Laboratories Ltd-India | 180 Tablets | LKR 200.00 | Tenofovir disoproxil registered . Justify need for Alafenamide. |
| 25 | 14.07.2021 | SPC/Leader Parma Agency Pvt Ltd | DHS/P/WW/81/20 | Methadone Hydrochloride Tablets 5 mg | Verve Human Care Laboratories -India | 32,000 Tablets | LKR 447.03 | BNF price Rs 13.35/= . Price quated LKR 447.03/= Not permitted at this price . |
| 26 | 19.07.2021 | Pharma Associates | DHS/P/WW/328/21 | Fludocortisone Tablets IP 100 mcg | Samarath Life Science Pvt Ltd-India | 175,000 Tablets | LKR 66.66 | Registered suppliers are available therefore Not permitted. |
| 27 | 20.07.2021 | Junaht International Pvt Ltd | DHS/RP/80/21 | Megestrol Acetate Tablets 40 mg | Regal Laboratories -India | 5,000 Tablets | LKR 192.86 | Both supplier & manufacturer not registered hence Not permitted. |
| 28 | 30.07.2021 | Tabrane Pharmaceuticals (PVT) Ltd | DHS/RP/CPU/0/090/2020 | Olaparib Capsules 50 mg | Drug International - Bangladesh | 130,470 capsules | LKR 269.00 | Advised NMRA to expedite registration . |
| 29 | 05.08.2021 | MSD | 2020/SPC/B/C/P/00640 | Misglustat Capsules 100 mg | Janseen Cilag International - Belgium | 730 caps | LKR 6890.00 | Permitted for 3 months (100 doses) although cost is very high LKR 1,240,200/= per month because it has been approved by SEC/Health according to file no : 2020/SPC/B/C/P/00640. |
| S No | Date of submission | Applicant | Quotation No./PO No./Tender No/Indent No. | Product | Supplier/Manufacture | Qty | Unit Price | Committee Decision |
| 1 | 19.05.2021 | George Steuart Health | INV NO: 0001/COV/21 | Remdesivir for injection 100mg/vial (5 mg/ml) | Aspiro Pharma Limited- India | 500 Vials | USD 60.00 | Not permitted. To be evaluated by Covid-19 Committee . |
| 2 | 20.05.2021 | SPC | LP/DHS/PM/3271/21 | Cocaine Hydrochloride Solution 10%, 2.5 ml bottle | Nixx Holdings (Pvt) Ltd/McCarty's Laboratories Ltd/T/A Martindale Pharmaceuticals -UK | 1000 Bottles | LKR 14250.00 | Not permitted. To get opinion from ENT Surgeons giving details of price . |
| 3 | 21.05.2021 | SPC | DHS/P/WW/721/21 | Povidone Iodine Cream 5% w/w , 15 g tube | Yaden International Pvt Ltd / Curis Lifesciences Pvt Ltd - India | 40,000 tubes | LKR 35.50 | Not permitted. MSD indicates it is not needed as oinment is available. |
| 4 | 21.05.2021 | Yaden International Pvt Ltd | DHS/P/WW/93/21 | Budesonide Controlled release Capsules 3 mg | Dagfon Pharmaceuticals Pvt Ltd - India / Eurek Life Science Pte Ltd - Singapore | 18750 Capsules | USD 5.80 | Not permitted. Registered product. Not an emergemcy use Medicine. BNF price is LKR 235/= per capsule . |
| 5 | 21.05.2121 | Ravilux Company (Pvt) Ltd | DHS/P/WW/555/21 | Morphine Sulphate ControlledRelease Tablets 30 mg | BDH Industries Limited - India | 450,000 Tablets | USD 0.18 | Not permitted. MSD has 18 months stock. |
| 6 | 21.05.2021 | Ravilux Company (Pvt) Ltd | DHS/P/WW/686/21 | Acyclovir Oral Suspension USP 200 mg/ 5ml, 125 ml bottle | BDH Industries Limited - India | 1000 Bottles | LKR 1226.37 | Not permitted. MSD not needed. |
| 7 | 21.05.2021 | Ravilux Company (Pvt) Ltd | DHS/P/WW/748/21 | Gentamicin 0.3%w/v + Hydrocortisone 1% w/v Ear Drops 10 ml | BDH Industries Limited - India | 3750 Vials | LKR 355.47 | Not permitted. MSD not needed. Stock available. |
| 8 | 21.05.2021 | Leader Pharma Agency (Pvt) Ltd | DHS/RP/410/2019 | Diazoxide Tablets BP 50 mg | Recipharm -Uppsala AB, Sweden. | 4900 Tablets | LKR 546.00 | Not permitted. To register as orphan drug . Six months stock available at MSD . BNF price LKR 130/= . |
| 9 | 25.06.2021 | Markss Healthcare Pvt Ltd | DHS/RP/375/2020 | Lamivudine film coated tablets 150 mg | Mylan Lboratories Ltd - India | 12,000 Tablets | LKR 32.08 | Not permitted. film coating not essential . |
| 10 | 02.06.2021 | SPC | DHS/P/WW/700/21 | Sodium Chloride 5% Eye Drops 10 ml " Hypet Eye Drops" | EDNA Medicals (Pvt) Ltd / The Schazoo Pharmaceuticals Laboratoroes Pvt Ltd - Pakistan | 18750 Bottles | LKR 685.27 | To implement decision of MEC. Not approved till then. |
| 11 | 08.06.2021 | Care Ring Pharma (Pvt) Ltd | LP/MSD/CPU-DHS/NRQ/361/T/2020 | Misglustat Capsules 100 mg | Janssen- Cilag International -Belgium | 730 Caps | LKR 6890. 00 | Not permitted. MSD to appoint a committee to decide on cost effectiveness. Will consider after MSD decision received by NMRA. Cost for one month / patient 100 mg tds 12,40,200/=. 200 mg tds 12,400,200/ =. |
| 12 | 31.05.2021 | SPC | DHS/P/WW/381/2021 | Phenytoin Sodium Tablets BP 100 mg | MAPS Pharma (Pvt) Ltd / Hiral Labs Ltd -India | 17,000,000 Tablets | LKR 1.28 | Rejected. Stock for 6 months available at MSD . Registered products are available . |
| 13 | 31.05.2021 | SPC | DHS/RP/70/20 | Chlorhexidine Gluconate Solution 20% w/v, 500 ml bottle | L.W Medicals/Unilab Chemicals & Pharmaceuticals Pvt Ltd -India | 21000 Bottles | LKR 701.06 | Not permitted. Local agent to register with NMRA. Ceyoka has already appied for registration of another product . MSD not essential urgenty . |
| 14 | 31.05.2021 | SPC | DHS/P/WW/568/21 | Chlorhexidine Gluconate Solution 4% w/v, 500 ml bottle | L.W Medicals/Unilab Chemicals & Pharmaceuticals Pvt Ltd -India | 27500 Bottles | LKR 501.61 | Not permitted. Local agent to register with NMRA. Ceyoka has already submitted an extension of registration for another product. |
| 15 | 15.06.2021 | Yaden International Pvt Ltd | LP-P-CPU/DHS/O/P/M/027/2021 | Docetaxel I njection 80mg/8ml & 20mg/2ml | Biozenta Lifesciences Pvt Ltd-India | 277 vials of 80mg/8ml vial & 348 vials of 20mg/2 ml | LKR 15700 & 14800 | Rejected. There are registered products. |
| 16 | 18.06.2021 | R N Vision Pvt Ltd | LP/MSD/CPU-DHS/RQ/050/(T)/2020 | Docetaxel TPGS injection 80 mg/8 ml vial & Docetaxel TPGS injection 20 mg/2 ml vial | Therdose Parma Pvt Ltd -India | 299 Vials & 437 Vials | Registered already. Hence WOR not needed. | |
| 17 | 08.06.2021 | Population Services Lanka | DHS/P/WW/235/20 | Misoprostol Tablets 200 mcg | Comed Chemical - India | 200000 Tab | Extend registration certificate . Stock available at MSD .Not permitted. | |
| 18 | 21.06.2021 | Care Ring Pharma (Pvt) Ltd | DHS/RP/245/2020 | Glycoppyronium Bromide Tablets 1 mg | Cotec Healthcare (Pvt) Ltd - India | 6250 Tablets | LKR 125 per tab | SPC indicates not needed. Manufacturer cannot supply. |
| 19 | 15.06.2021 | Pharma Associates | LP/DHS/AMS/3278/2021 | Phosphate Tablets 500 mg | Osaka Pharmaceuticals Pvt Ltd - India | 37500 Tablets | LKR 165 per tab | Not permitted. Negotiate price first for a WOR decision, BNF price LKR 43/=. |
| 20 | 17.06.2021 | The Esses Pharmacy (Pvt) Ltd | DHS/RP/247/2016 | Dehydrated Alcohol (Ethanol) injection USP 5 ml ampoule | Martindale Pharmaceuticals-UK | 20 Ampoules | LKR 49500 Per vial | Cardiologist to justify need. Indicate price in letter.Not permitted. |
| 21 | 10.06.2021 | Leader Pharma Agency (Pvt) Ltd | DHS/P/DQ/659/18 | Human Varicella- Zooster Immunoglobulin 250 mg solution for injection- , 100 IU/ML | Bio Products Laboratory Ltd (BPL)-UK | 90 Vials | LKR 262438.88 | Not permitted. Stock available for five months. |
| 22 | 16.06.2021 | Tabrane Pharmaceuticals (PVT) Ltd | DHS/RP/CPU/O/053/2021 | Olaparib Capsules 50 mg | Drug International (Pvt) Ltd- Bangladesh | 26540 Caps | LKR 279.00 | Expedite registration.Not permitted as WOR. |
| 23 | 17.06.2021 | Leader Pharma Agency (Pvt) Ltd | DHS/P/WW/121/2021 | Methionine Tablets 500 mg | Verve Human Care Laboratories -India | 40000 Tablets | LKR 168.39 | Not needed as N-Acytayl cysteine is available. |
| 24 | 16.06.2021 | Oreo Bio Parma (Pvt) Ltd | DHS/P/WW/453/20 | Ethinyloestradiol Tablets BP 20 mcg | Solitaire Pharmacia Private Limited- India | 3500 Tablets | LKR 520. per tab | Not permitted. Register if this strength is needed. |
| 25 | 16.06.2021 | Orexo Bio Parma (Pvt) Ltd | DHS/P/WW/28/21 | Cyclophosphamide Tablets BP/USP 50mg | Health Bio Tech Ltd - India | 300,000 Tblets | LKR 29. per tab | Company to submit data for evalvation of efficacy , Safety & quality as recommended by SPC . |
| 26 | 21.06.2021 | Tabrane Pharmaceuticals (PVT) Ltd | DHS/RP/CPU/B/054/2021 | Valpatasvir 100 mg + Sofosbuvir 400 mg tablet | Drug International (Pvt) Ltd- Bangladesh | 219 Tab | LKR 699.0 per tab | Not permitted. Register product under Orphan drugs. |
| 27 | 15.06.2021 | Care Ring Pharma (Pvt) Ltd | LP/P-CPU/DHS/O/E/002/2019 | Panitinumab Injection 100 mg/5 ml | Amgen INC-USA | 144 Vials | LKR 155,500 per vial | MSD indicated that item is not needed.Not permitted. |
| 28 | 17.06.2021 | Markss Healthcare Pvt Ltd | LP/DHS/IG/3267/20 | Abacavir 300mg tablet | Mylan Laboratories Ltd - India | 31080 tab | LKR 118.33 | Not permitted. Buy from registered source. |
| 29 | 16.06.2021 | SPC | DHS/AMS/317/2020 | Rifampicin Capsules BP 150 mg | Ravilux Company Pvt Ltd/BDH Industries Ltd-India | 300000 | Not needed as it is not registered-Anti TB Campaign. | |
| 30 | 25.06.2021 | Markss HLC (Pvt) Ltd | DHS/RP/CPU/B/093/2020 | Omalizumab Injection 150mg/ml , 1 ml pre filled syringe | Vetter Pharma- Germany | 26 pre filed syringes(PFSY) | LKR 38,000.00 | Not permitted. MSD to submit evaluation report from committee for cost effectiveness. |
| 31 | 25.06.2021 | Markss HLC (Pvt) Ltd | DHS/RP/CPU/B/088/2020 | Omalizumab Injection 150mg/ml , 1 ml pre filled syringe | Vetter Pharma- Germany | 18 pre filed syringes(PFSY) | LKR 38,000.00 | Not permitted. MSD to submit evaluation report from committee for cost effectiveness. |
| 32 | 21.05.2021 | Markss Healthcare Pvt Ltd | DHS/P/WW/741/21 | Lamivudine tablets 150mg | Mylan Laboratories Ltd - India | 72000 Tablets | LKR 14.66 | Permitted Only for this shipment. Register for next requirement . |
| 33 | 17.06.2021 | Ravilux Company (Pvt) Ltd | DHS/P/WW/325/2021 | Dexamethasone Tablets 4 mg | BDH Industries Limited - India | 3,75,000 Tablets | Permitted owing to Covid & register supplier is unable to supply according to SPC. | |
| 34 | 17.06.2021 | Ravilux Company (Pvt) Ltd | DHS/P/WW/324/2021 | Dexamethasone Tablets 8 mg | BDH Industries Limited - India | 90,000 Tablets | Permitted owing to Covid & register supplier is unable to supply according to SPC. | |
| 35 | 15.06.2021 | George Steuart Health | LP/DHS/AMS/3284/20 | L-Asparaginase for injection 10,000 KU Vial | United Biotech Pvt Ltd - India | 1900 vials | LKR 6800.00 | Registered product. Now registration route has been changed. Hence permitted. Expidite registration. |
| 36 | 12.05.2021 | Anti Leprosy Campaign | INV NO: 9188602504 | Anti Leprosy Drugs | World Health Organization | Listed Qty | Permitted- Free of Charge | |
| 37 | 11.05.2021 | NPTCCD | OD NO: 20201329 SO | Anti TB Drugs | IDA Foundation - Netherlands | Listed Qty | Permitted- Free of Charge | |
| 38 | 16.06.2021 | STD/AIDS | Inv no: 187000936 | Emtricitabine 200 mg +Tenofovir Disoproxil Fumarate 300 mg Tablets | Hetero Labs Limited -India | 25920 Tablests | Permitted- Free of Charge | |
| 39 | 21.06.2021 | Anti Leprosy Campaign | Ref-UCN/NTD/STO | Anti Leprosy Drugs | World Health Organization | Listed Qty | Permitted- Free of Charge | |
| 40 | 08.07.2021 | MSD | CA/108424D/0089/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 41 | 08.07.2021 | MSD | CA/108424D/0097/001 | Radioactive Materials- Sodium Iodine (131) solution & caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 42 | 08.07.2021 | MSD | CA/108424D/0083/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 43 | 08.07.2021 | MSD | CA/108424D/0085/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 44 | 08.07.2021 | MSD | CA/108424D/0087/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 45 | 08.07.2021 | MSD | CA/108424D/0086/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 46 | 14.07.2021 | MSD | CA/108424D/0094/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 47 | 14.07.2021 | MSD | CA/10842D/0084/001 | Radioactive Materials- Sodium Iodine (131) caps | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge | |
| 48 | 14.07.2021 | MSD | CA/108424D/0096/001 | Radioactive Materials- Sodium Iodine (131) caps & liquid | Ezacibasi-Monrol Nuclear products Co- Turkey | Listed Qty | Permitted- Free of Charge |




