Documents for Obtaining Sample Import License
- Application form
- Business Registration Certificate
- Valid Original Letter of authorization (as a Sole Agent LOA or Tabulated LOA)
- Declaration from the manufacturer stated no more Importers registered in Sri Lanka.
- Declaration from the importer.
- Product label (Art work/Scanned commercial label)
(Separate application should be submitted for different manufacturers)
Data Requirement for Preparation of Cosmetic Application for Registration
General documents / requirements
- Fulfilled “Schedule I form A & B” application [75 KB]
- Copy of sample import license.
- Free sale certificate or certificate to foreign government issued from relevant health authority of country of origin or certified by Sri Lankan Embassy of country of origin or foreign affairs
- Letter of authorization from the manufacturer appointing the Market Authorization Holder
- Certificate of analysis report of final product.
- Composition: the ingredient list by their chemical name with CAS number , function and include their exact quantities.
- Fully packed samples (two) of cosmetics in the form that is intended to be marketed (including Lot no., Man. Date, Exp.date, Manufacturer’s & Importers details)
- Documents should be in English, in a legible font size, printed in one side A4 and submitted in a hard file cover, and all pages should be numbered from top to bottom and vice versa with an index.
- Separate applications should be made in respect of each cosmetic to be registered. [i.e. products containing different colors, different brands etc.]. Products of foreign manufacturers should be submitted through a Marketing authorization Holder
*Applications made without these requirements will not be accepted.
Validity Period of Registration
- The Provisional Registration for a period of one year (or two) will be issued for first time registration and is specified in the certificate.
- The Full Registration of a product is valid for a period of five years and is specified in the certificate.
- When additional data are requested, the applicant will have to furnish additional information requested by the authority within 3 months to facilitate further evaluation.
- If the product is rejected, the market authorization holder will be able to appeal for registration.
Renewal of Registration
- Application for renewal should be made before six months from the date of expiry of registration.
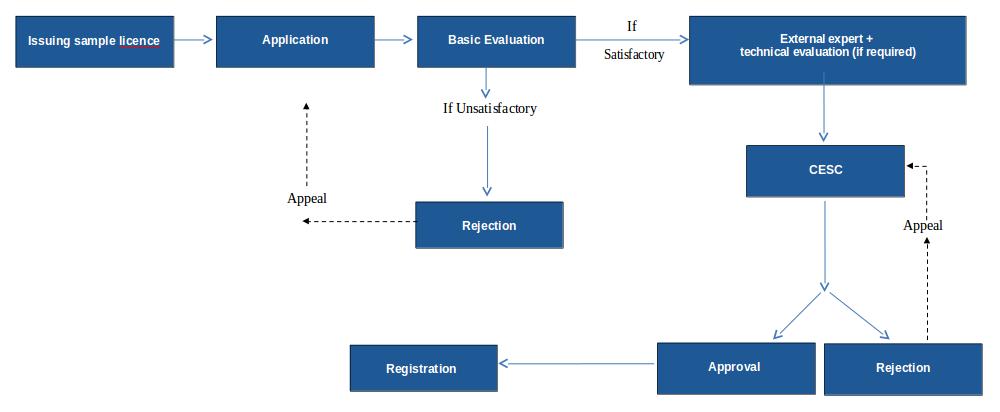
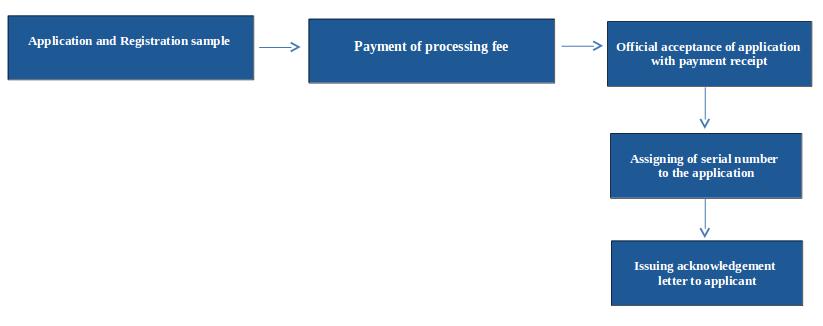
Flow Chart of Registration Process of Cosmetic is as Follows
Steps involved for registration of cosmetic
Obtaining sample import license
Submission of registration application
* “We are now in the process of revising the existing guidelines on regulation of cosmetics in Sri Lanka. To ensure the safety, quality and cost effectiveness of the cosmetics and to keep up with the existing guidelines in other countries of the region .We may ask you additional information during the process of registration of cosmetics. Please bear with us”.