Procedure for obtaining Marketing Authorization for Medicines
Step 1 - Approval of new manufacturer for registering medicines
An application to be submitted for approval of the manufacturing site. If the manufacturer is from a foreign country, a local company authorized to represent the manufacturer should be appointed and all applications should be furnished through the aforesaid local agent.
Upon approval of the manufacturing site, an application for registration of the medicines which are manufactured by the pertinent manufacturer should be submitted.
Step 2 - Registration of Medicines
Types of Application : Medicines are broadly subdivided into the following categories for the submission of applications for approval.
New Molecule Entity (NME)
A Chemical moiety which has not been previously registered in Sri Lanka, including an active ingredient of a salt, an ester or complex of a previously approved Chemical moiety.
New Dosage Form (NDF)
Any physical form of a registered medicine in Sri Lanka other than the available registered forms.
New Combination Product (NCP)
A new combination product is a formulation of two or more medicines in a single dosage form which has not been previously registered in Sri Lanka
New Product (NP)
Any new product of already registered medicine in Sri Lanka
Biological Products
Vaccines & Sera (Guidelines for Handling and Storage of Vaccines in the Private Sector)
Plasma products
Bio-technological products
Other biological products
The applicant for marketing authorization (i.e. the local manufacturer or the local importer representing the manufacturer) should submit an application (Documents required) in the format given in the Schedule IV, Form A of CDD Regulations with drug samples to the National Medicines Regulatory Authority (NMRA). Prior to submitting the application, a sample license needed to be obtained from the NMRA in order to facilitate customs clearance when importing the registration samples.
Guidelines on Evaluation of Similar Bio therapeutic Products (SBPs)
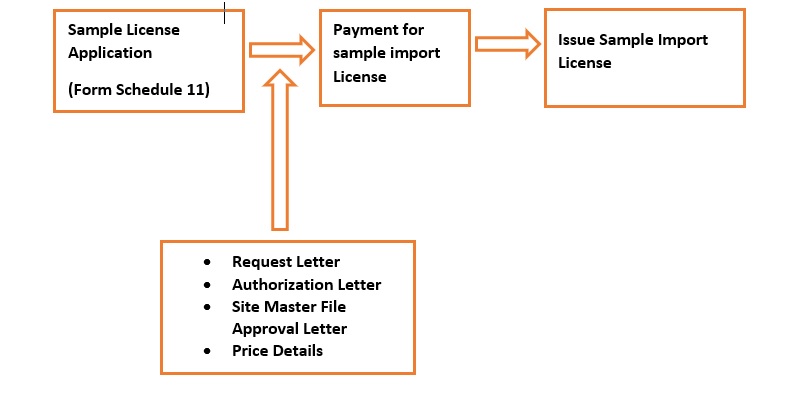
Obtaining Sample Import Licenses
An applicant for marketing authorization of medicine is required to submit minimum two packs representative of commercial packs he intend to market in Sri Lanka, along with the application for marketing authorization. If the manufacturer is from overseas, a sample license from NMRA would be required in order to facilitate customs clearance when importing such registration samples.
The following documents are required to be furnished by the applicant along with the application (Schedule XI) for import of registration samples.
- Request letter for a sample license by the applicant
- Letter of authorization by the manufacturer indicating the applicant as his local agent
- Letter of approval for the manufacturing site by NMRA (CP approval letter)
NMRA reserves the right to refuse to issue a sample license to a particular product. Currently NMRA does not accept applications if there are twenty or more registered products of the particular item.
Once the applicant obtains the sample license, he is required to abide by the condition of the license, which is specified in relevant regulations. Particularly, the applicant should maintain all records pertaining to goods imported on such sample licenses.
Import of registration samples
Submission of Registration Application
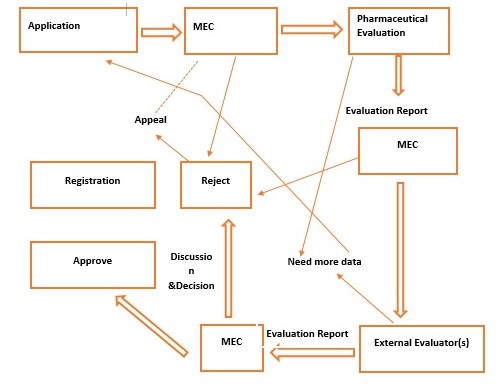
Figure 1: Registration of NP Applications
The certificate of registration is valid for 5 years. Under specific circumstances (e.g. when the drug is a new chemical entity, the manufacturer is new to this country) a provisional registration will be issued first, which is valid for one or two years.
A renewal application in Schedule IV form A and other documents required should be submitted 6 months before expiration of the existing registration.
Any changes (variation) of registered medicine should be informed to NMRA, NME, NDF, NCP
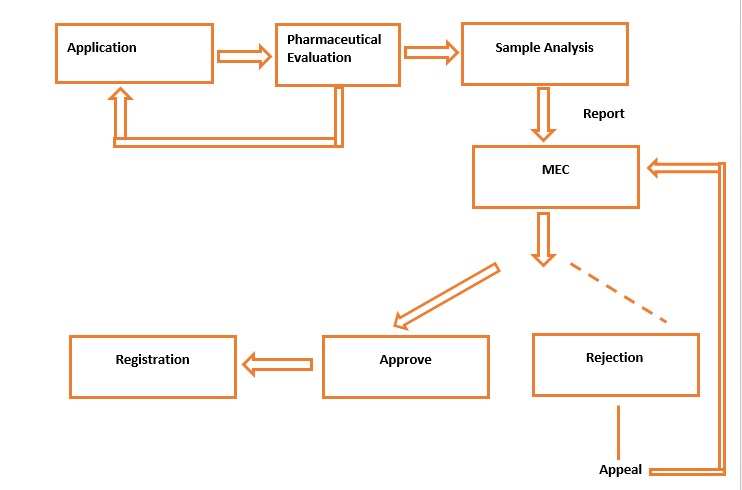
Figure2 : Registration of NMEs, NDF & NCPs